What is Nanoscience?
Nanoscience and nanotechnology are terms that are heard more and more these days. They are used to describe a vast array of research and engineering. Essentially, nanoscience deals with material on very small length scales down to the level of single atoms or molecules. One nanometer is 10-9m and an atom is approximately 0.1 nm in size. Fifty years ago no one had ever seen an individual atom but today we are starting to arrange them in the patterns of our choosing. Nanomaterials are composed of fundamental building blocks which are nanometers in size. These nanomaterials have unique properties that are only now being studied. These properties can be harnessed to create exciting new technologies that will benefit the environment.
In the catalyst section, we introduced the concept of a surface being used to help speed a reaction. Reactants come into contact with a surface and get converted to products. To increase the probability of the reactant molecules colliding with the surface we need to increase the amount of exposed metal surface. We can increase the metal surface area by spreading the metal over a support structure. In practice we make a very fine dispersion of metal nanoparticles which are placed on an oxide support. For a given volume of metal, making a fine dispersion of nanoparticles dramatically increases the available metal surface area on which catalytic reactions can take place. These metal nanoparticles may also be highly reactive. This demonstrates one way in which nanomaterials can make a big impact on environmental technologies such as catalytic converters, fuel cells, and alternative fuels.
The relationship between particle size and surface area is illustrated below. Taking the same volume of metal and making many nanoparticles dramatically increases the surface area.


For the math wizards :)
Given the equations for surface area and volume below,
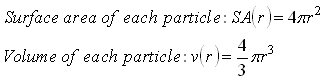
One may solve for total surface area as a function of particle radius (r), assuming
a constant total volume V and number of particles
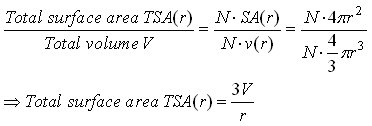
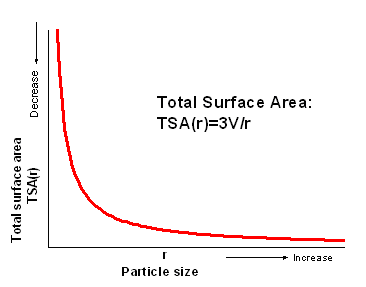
Conclusion: It is clear that the total surface area will decrease as particles size increases
with a constant total volume
Next: Electron Microscopy
Funded By

|