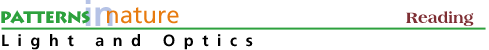
The behavior of all these photons are basically the same: the photons travel at the same velocity in vacuum with energies that can vary over ten orders of magnitude from the very low energies of radio waves to the very high energies of gamma rays emitted from the nuclei of radioactive atoms.
If light is imagined as a flow of particles, the particles are called photons with each photon carrying a discrete packet of energy. For a beam of fixed energy photons, the intensity of the beam depends the number of photons per second. Light can also be described as waves with the distance between waves, the wavelengths, inversely proportional to energy. The low energy radio waves have wavelengths of meters and the high energy x-rays can have wavelength of a millionth of a meter or less.
Light can be described as a particle (photon) or a wave (electromagnetic wave). The electromagnetic wave can be pictured as oscillating electric and magnetic fields that move in a straight line at a constant velocity (the speed of light).
Sound waves are not part of the electromagnetic spectrum and are not identified with photons. Light travels in vacuum - from the sun to the earth, for example - whereas sound, which is a vibration of air molecules, can not exist in vacuum. Both do have wave behavior but the mechanisms are different.
Light described as photons allows a visualization of the absorption - disappearance - of light. In the photoelectric effect shown in Fig 1 below, a photon incident on a metal surface transfers all its energy to an electron and disappears while the electron - now containing the energy of the photon - leaves the surface of the metal.
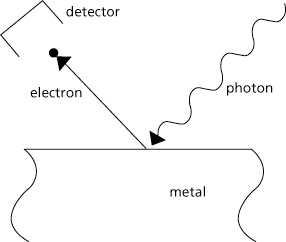
Figure 1. In the photoelectric effect a photon incident on a surface (here, a metal surface in vacuum) transfers its energy to an electron, which leaves the surface and is detected. The photoelectric effect demonstrates the particle nature of light.
This photoelectric effect was described by Albert Einstein in 1905 as the interaction of a photon with an electron in a solid.
The photon has only energy and no mass. When the photon gives up all its energy to an electron the photon disappears. The electron on the other hand, has mass and charge - electrons carry the electrical current in wires.
Photons at extreme energies (millions of electron Volts of energy) can create electrons. Here, the high energy photon, or gamma ray, produces an electron and its anti-particle, the positron (positively charged with the same mass as the electron). The gamma ray disappears (Fig 2) in the formation of electron and positron, called pair production.
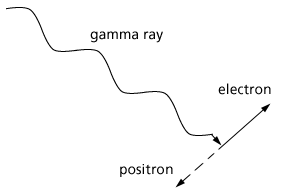
Figure 2. The formation of an electron positron pair by the annihilation of a gamma ray.
The two oppositely charged particles (electron and positron) can recombine with each other and disappear with the creation of a photon.
In the realm of visible light the photons are absorbed and disappear in giving their energies to outermost atomic electrons that are only held in place by energies of a few electron volts. The figure below shows electrons occupying energy levels (vertical scale) and an incident photon
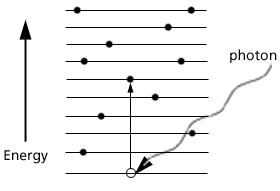
Figure 3. Photons absorbed in a solid by delivering all its energy to an electron which moves to a higher energy level.
losing its energy and disappearing by moving an electron to a higher energy level.
In the discussion of reflection and refraction it was pointed out that the path of light was reversible. A photon in air incident on glass would be refracted at the air/glass boundary. The same path would be followed in reverse for a photon in glass incident on air and refracted at the glass/ air boundary. A similar reversibility is found in the interaction of the photons and electrons. In Fig 3, if the energetic electron returns to its original position, the energy given up in the transition from higher to lower energy states appears in the form if a photon. The same reversibility is true in pair production (Fig 2). If an electron and positron combine together and disappear, there energy appears as a gamma ray.
This then is the life cycle of a photon as shown in Fig 4. Life and death (creation and disappearance) are contained in the interaction of electrons and photons. The electrons are charged particles with mass: they change energy but do not disappear. The photons have no mass nor charge: they appear and disappear.
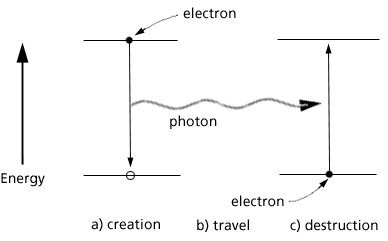
Figure 4. The life cycle of an electron: a) the electron loses energy and creates a photon which b) travels in space where it encounters c) an electron and disappears.
Vision is also connected with the life cycle of the photon. We detect objects by the reflection of light from them. The reflected light enters the eye and triggers the photoreceptors which sends a signal to the brain. The photon disappears in giving its energy to the photoreceptor.
Quite the opposite view was held by Plato and Euclid. They believed that the eye emitted rays of light (Fig 5) which senses the objects in front of them. This incorrect model of vision today is still displayed in the x-ray vision of superman. He projects x-rays, and somehow senses the objects in front of him.
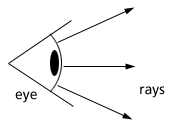
Figure 5. The eye sends out visual rays to sense what is in front of it. This is a misconception of the role of the eye.
Go to the Readings Page
Go Back to the Previous Page
Page authored by ACEPT W3 Group
Department of Physics and Astronomy, Arizona State University, Tempe, AZ 85287-1504
Copyright © 1995-2000 Arizona Board of Regents. All rights reserved.