
Activity 10
Introduction
Review the Reading Optical Spectroscopy and Neon Lights and review your Lab One, Module A-8.Procedure
Background
When an element is bombarded with high-energy electrons in an X-ray tube, it also emits characteristic X-rays with sharply defined energies. Figure 1 shows the X-ray
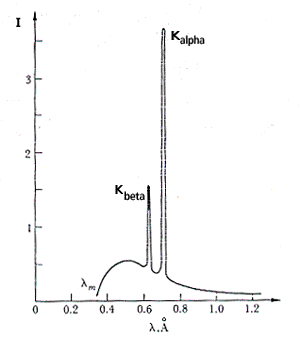
Figure 1. X-ray spectrum of molybdenum showing the Kalpha and Kbeta lines superimposed on a continuous spectrum. The wavelengths of the lines are characteristic of the target element.
spectrum from molybdenum showing two peaks at wavelengths between 0.6 and 0.8 Ångstroms (Å). The Kalpha and Kbeta identify the electron transitions where the electrons move into the innermost electron level (the K-shell). The characteristic peaks (termed line spectra) have specific energies that can be identified with each element. Measurement of the energies of the line spectra energies allows one to identify the elements emitting the X-rays.
Most laboratories with a scanning electron microscope (SEM) have an X-ray spectrometer attached for the measurement and detection of X-rays. These instruments that measure X-ray energies contain liquid-nitrogen (LN2) -cooled detectors and can be easily noticed by the cylindrical dewar (similar to a thermos bottle) about 8 inches in diameter and 12 inches tall. If you are in a laboratory with an SEM, see if it has an instrument for measuring the energy of X-rays.
Go to the Reading on The Electron
Page authored by the ACEPT W3 Group
Department of Physics and Astronomy, Arizona State University, Tempe, AZ 85287-1504
Copyright © 1995-2000 Arizona Board of Regents. All rights reserved.