|
Organometallic Reagents |
Carbon-Carbon Bonds
|
|
|
|
|
|
|
1 Carbon-Metal Bonds |
• We previously looked at the following two disconnections…
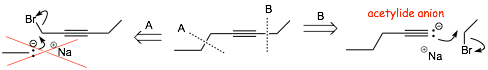
• B is better since it generates a useful ACETYLIDE synthon/reagent
• The acetylide anion is a simple organometallic reagent, i.e. an organic structure with a bond to a metal
• In this case the carbon metal bond is essentially pure ionic
![]()
Q. Suppose we HAD to do disconnection A above, how can we make a reasonable carbon synthon such as that in A if we don't have an sp A.O. to stabilize the electrons? An ionic bond to a sodium cation will not work if the hybridization has higher p character than sp.
A. We must "temporarily" stabilize the non-bonding electrons on the carbon another way, we can out them into a weak bond to a metal atom
Compare BONDING to different metals for sp3 hybridized carbon
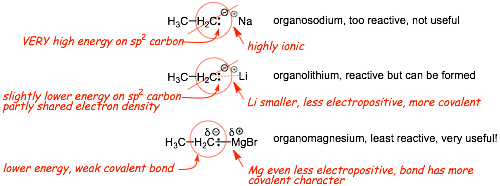
• weak covalent bonds with a lot of ionic character mean that the electrons are still relatively high in energy and quite reactive
• both organolithiums (alkyl and aryl lithiums) and lithium compounds and organomercury compounds Grignard reagents) can be made for carbons with ANY hybridization, including sp3 and and are thus very useful
• alkyl and aryl lithiums are more ionic and tend to be very strongly basic, but they are still nucleophilic and can be used to make carbon-carbon bonds
• organomercury compounds in the form of Grignard reagents tend to be the most useful nucleophiles
ORGANOMETALLIC reagents are useful carbon anion synthons/reagents
Organolithium reagents

Grignard reagents

• the reaction is between an akyl or aryl halide (often bromide) with metallic magnesium in an ether solvent
• solvents for Grignard reaction must be ethers, two are most commonly used….
![]()
• as reagents…..
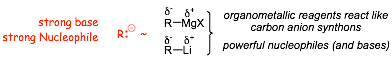
• Grignard, less reactive, easier to handle, although restricted to ether solvents however
• Organolithiums, more reactive, harder to handle, are VERY STRING BRONSTED BASES (see later), can be used in different solvents
Reactions of Organometallics With Water
• Not usually a useful reaction, but one that is important to know
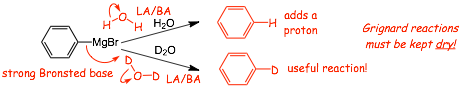
• this is hydrolysis of a carbon-metal bond, we have seen this before, and will see again (and again..)
• THIS is the reason that reactions of organometallic reagents have to be performed in the absence of ALL water (apparatus and solvents must be dried)
|
2 Nucleophilic Addition Reactions |
Example Problem:
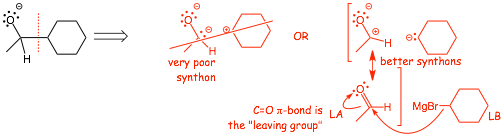
• the positive synthon (with the positive charge) is stabilized by simply generating a C=O bond, i.e the positive synthon is simply an aldehyde, in this case the C-O pi-bond is the "leaving group"
• the non-bonding electrons in the negative synthon are stabilized by putting them into a weak carbon-metal bond
• we now have excellent synthetic equivalents (actual reactants), an aldehyde and a Grignard reagent
Reactions With aldehydes, ketones and epoxides
• makes a new C-C bond (and an alcohol, similar to acetylides), VERY IMPORTANT REACTION
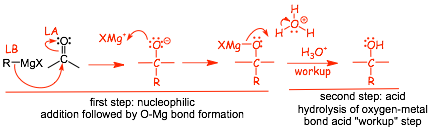
• two step reaction, the first is Grignard addition to the C=O bond (followed by addition of XMg+ to the oxygen)
• the second "acid workup" step is very different from, for example, the acid catalyzed addition of water to an alkene, which requires a lot of acid, heat and time. Acid workup usually means using a fairly dilute acid solution for a short amount of time, the "reagent" H3O+ can mean very different things!
• This is important because unlike the acetylide reaction, the reagents can be prepared from a HALIDE, and halides are much more readily available and generally useful than alkynes
Examples
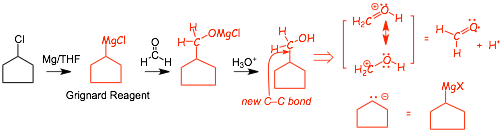
• NOTE: Retrosynthetic analysis in this case converts the positive synthon into a C=O structure PLUS a proton (H+), the proton is supplied in the second acid workup step in the form of H3O+

NOTE: Grignards can be made at sp3 AND sp2 hybridized carbon atoms
Example with epoxide
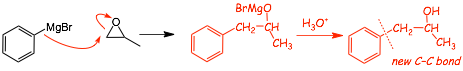
• the Grignard attacks the least-substituted side of the epoxide (seen before), for steric reasons
• the oxygen on the carbon ADJACENT to that in the new C-C bond, characteristic of addition to an epoxide
Example With Esters: Addition/Elimination (again)
Recall: Reaction of LiAlH4 with an ester

• Hydride addition to an ester, occurs TWICE in an ADDITION/ELIMINATION mechanism followed by ADDITION
• -OR is the leaving group, and while not particularly good, elimination is favored by entropy, AND, the intermediate also has a negative charge on oxygen
• -OR will only be a leaving group when the starting reactant has high chemical potential energy in the from of reactive electrons, usually as an ANION, AlH4- in this case, OR, as a Grignard reagent, see below
Similarly: Reaction of Grignard with an ester
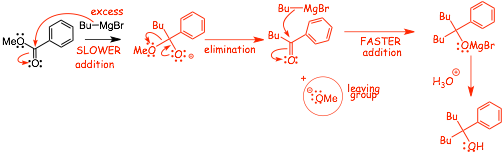
• The Grignard with an ester reaction occurs by ADDITION/ELIMINATION mechanism followed by ADDITION
• -OR is the leaving group, and leaves for the reasons given above for hydride
• The Grignard reacts SLOWER with the ester than it does with the ketone
• This turns out to be important, because the product of the Grignard reaction with the ester reacts FASTER, which means any attempt to use only 1 Equivalent of Grignard is doomed to fail, since as soon as the ketone is formed it will consume Grignard before it can react with the ester
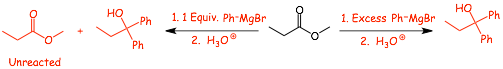
• Therefore you must specify Excess (XS) Grignard in these reactions with esters
• Recall, we previously learned that strong nucleophiles such as NaBH4 and LiAlH4 react slower with esters than they react with aldehydes and ketones, this is why you need the stronger nucleophile LiAlH4 to reduce an ester
• This is because the C=O group in the ester is not as electrophilic as the C=O group in an aldeyde or ketone
• We can consider the C=O to be a small pi-system that has substituents with different donating abilities

• the stronger donating -OMe substituent decreases the reactivity of the ester with respect to the ketone
Example With Acid Chlorides: Addition/Elimination (again)
Acid chlorides have an even better leaving group than esters, we expect addition/elimination also, and this is exactly what happens

Grignards do NOT do SN2 reactions with most alkyl halides
• Unlike the acetylide anion, Grignards do not do SN2 reactions with most alkyl halides (allylic halides are an exception, as we will see later….)
Acetylide SN2 reaction with a halide….
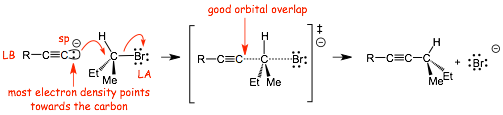
• The Lewis basic electrons in the acetylide anion are in a small sp2 hybrid A.O. that "points" directly towards the carbon of the alkyl halide, the partial bond in the transition state is strong, the reaction is fast
• The acetylide anion is a strong base AND a very strong nucleophile (remember, the definition of nucleophilicity is based on reaction kinetics, how fast the reaction goes, not just on how strong a bond it can make)
Grignard SN2 reaction with a halide….
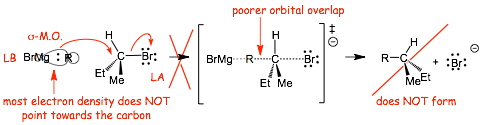
• The Lewis basic electrons in the Grignard reagent are in a larger sigma-bonding M.O. and most of the elctron density does NOT "point" directly towards the carbon of the alkyl halide, the partial bond in the transition state is weak, the reaction is slow
• The Grignard reagent is a strong base BUT a weak nucleophile (remember, the definition of nucleophilicity is based on reaction kinetics, how fast the reaction goes, not just on how strong a bond it can make)
• We will find that Grignards CAN do SN2 reactions in special cases, specifically in the halide is allylic or benzylic due to stabilization of the transition state in that case (see later)
• In advanced organic chemistry classes you will also find that the reaction of Grignards with C=O bonds is actually a bit more complicated than we pretend it is here! But our model of these reactions is sufficient to explain everything we need for this course, and in organic chemistry we tend to use the simplest model that works.
|
3 Organometallics in Retrosynthesis |
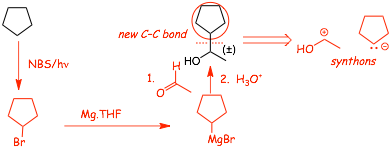
Example 1
Example 2
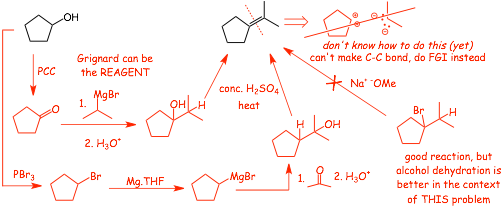
• The last reaction is CAN'T be making the required C=C bond because we have not learned how to make a C=C bond yet, therefore the last reaction HAS TO BE making the alkene from another functional group, i.e. a functional group interconversion (FGI)
• we can make an alkene by E2 elimination of an alkyl bromide, OR, by dehydration of an alcohol
• elimination of the bromide is a good reaction, but is less helpful in the context of this overall synthesis problem, because if we use an alcohol that will help us to solve the C-C bond making problem in the previous step,. Since alcohols are often formed in Grignard reactions, therefore in this case dehydration of the alcohol is best
• but there are TWO possible alcohols that can be dehydrated, which one to choose? It turns out that you can use either, in many multi-step synthesis problems there is MORE THAN ONE possible solution, this is one of those
Example 3
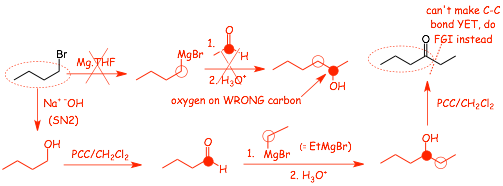
• The last reaction is CAN'T be making the required C-C bond because it is to an sp2 hybridized carbon that is part of a ketone, we don't know how to do that, therefore the last step has to be making the ketone from another functional group, i.e. a functional group interconversion (FGI)
• we can make a ketone from an alcohol, which is a good idea because we know that we can make C-C bonds that have alcohols in the reaction products when using a Grignard reagent
• be CAREFUL when constructing Grignard reactions, make SURE that you have the -MgBr and the C=O on the correct fragments to get the -OH on the correct carbon after the reaction is complete
|
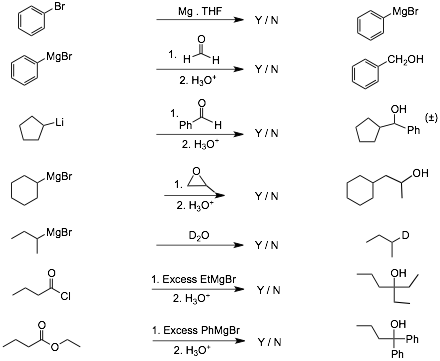
4 Organometallic Reagents : Summary of Reactions |
Do NOT start studying by trying to memorize the reactions here!
Work as many problems as you can, with this list of reactions in front of you if necessary, so that you can get through as many problems as you can without getting stuck on eth reagents/conditions, and so that you can learn and practice solving reaction problems. Use this list AFTER you have worked all of the problems, and just before an exam. By then you will have learned a lot of the reagents/conditions just by using them and you will only have to memorize what you haven't learned yet. Then do the following:
• Cover the entire page of reagents/conditions with a long vertical strip of paper, see if you can write down the reagents/conditions for each reaction, check to see which you get correct, if COMPLETELY correct, circle Y, if incorrect or even slightly incorrect, circle N. In this way you keep track of what you know and what you don't know.
• Keep coming back to this list and so the same thing only for those reactions you circled N, until all are circled Y.
Knowing the reagents/conditions on this page is INSUFFICIENT to do well on an exam since you will ALSO need to recognize how to use and solve reaction problems in different contexts, this page ONLY helps you to learn the reagents/conditions that you have not YET learned by working problems.
Obviously we like to minimize memorization in a class that is designed to help you understand organic chemistry, but you can’t work everything out from first principles, and there is nothing wrong with a little bit of memorization. There is a reason that it is useful to "just know" some material. Material that you just know can be used more quickly and accurately than material you have to "work out". This is why we memorize multiplication tables, for example.
WARNING: Carbon-carbon bond forming reactions require lots of practice and occur in many more contexts than summarized here!