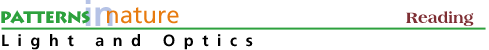
Light and Electrons
"There are three stages in the life of a light beam: it is created, it travels through space, and it is destroyed...light is created and destroyed only via its interaction with matter, from glowing gases in the sun to rhodopsin in the eye." (Michael Sobin in "Light", p. 73, University of Chicago Press,1987). It is the interaction of light with electrons that is responsible for its creation and destruction. Here we will use photons for light because it is the particle-like description that is easier to visualize rather than wave-like behaviour.Electrons in matter can be described as occupying energy levels. Electrons can be raised to higher energies by absorbing energy from a photon (destroying light) or move to lower energies by giving off a photon (creating light) whose energy is equal to the difference in energy between the two levels. (See the Reading Electrons in Atoms) Figure 1 shows two electron levels separated in energy by 2 electron volts (E = E2 - E1 = 2 eV).
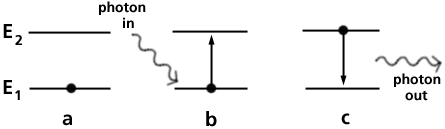
Figure 1. Two electron energy levels in a solid: a) electron occupying the lower level and the upper level empty, b) photon is absorbed by the electron which moves to the upper level, c) photon is created by electron moving from upper to lower level with energy = E2-E1
An incident photon (orange light of 620 nanometers or 2 eV) gives up its energy (and vanishes) to the electron which moves to the upper level (Fig 1b). In Fig 1c, an electron in the upper level moves to the lower level by giving up an energy of 2 eV and emitting orange light. Energy is conserved.
Electrons in atoms and in matter - from gases to solids - are held in place and require a boost in energy to remove them. In atoms the negatively charged electrons are held (bound) by the positively charged protons. From the quantum theory, we know that the electrons are bound to the atom with fixed energies, the binding energies. In the semiconductor element silicon the binding energies for the innermost electrons are nearly 2000 electron volts (eV) - more exactly, 1.839 kiloelectron volts (keV). This means that an energy of at least 1.839 keV must be given to an innermost electron to remove it from the silicon atom. These 1 keV energies lie in the x-ray portion of the spectrum.
The outermost electrons in silicon have a binding energy of 3 electron volts. This is at the upper end of the visible light energy spectrum. Violet light has an energy close to 3.1 eV with a wavelength of 400 nanometers (Energy in electron volts equals 1240 divided by the wavelength in nanometers). In solid silicon the outermost electrons are shared between atoms and these electrons are bound to the solid by energies of 1.1 electron volts. This means that visible light is absorbed by silicon and infrared light of energy less than 1.1 eV and wavelengths greater than 1100 nanometers can pass through silicon without absorption (see the Reading Infrared and Ultraviolet Light).
Optical Light Emission
Light is emitted over the whole energy region of visible light byi the heating of a tungsten filament in a light bulb (see the Reading Sources of Light). With electric discharge in gases light can also be produced in a spectrum of narrow (also called sharp) lines distributed in energy across the visible spectrum. Each line is characterized by the element atom which emits the light. Sodium emits two lines fairly close together in the yellow region of the spectrum, cadmium emits a strong red and a strong green line, and mercury emits several strong lines. Figure 2 shows the energy-level diagram for mercury and the wavelengths of light emitted by the transition of electrons from upper to lower levels.
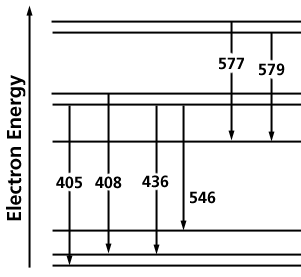
Figure 2. The energy levels in mercury which produce the allowed electron transitions which produce visible light. The wavelengths are given in nanometers. The strong green line at 546 nanometers is used to calibrate spectrometers.
Optical Spectroscopy
Optical instruments are used to measure the intensities and wavelengths of the visible region of the electromagnetic spectrum. A spectroscope is a "spectrum-observing" instrument and a spectrometer is for "spectrum measurement". A spectrometer measures the wavelength of light.The refraction and dispersion of light through a prism produces a spectrum of light from red to violet. Figure 3 shows a simple prism spectrometer for
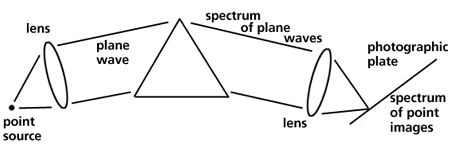
Figure 3. A simple prism spectrometer
examining a light source with a focusing lens to produce parallel light (plane waves) which is dispersed by the prism. The spectrum of plane waves can be focused onto a photographic plate. The plate must be tilted to keep all the colors in focus because of the change in focal length of the lens with wavelength.
As part of the patterns in nature you have used or made an optical spectrometer based on a diffraction grating (a grating is contained in the Optics Kit). The incident light passes through a slit, through the grating, and into your eye. The eye is an essential part of the instrument for the eye collects the light from the grating and focuses the many colors on the retina. The eye sees these colors superimposed on the wavelength scale of the spectrometer. Figure 4 shows a schematic of the Project STAR Spectrometer (Learning Technologies, Inc., 40 Cameron Ave., Somerville, MA 02144).
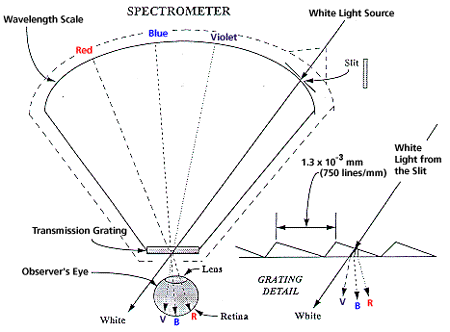
Figure 4
Neon Lights
In the mid-1800's, it was found that an evacuated tube - a glass cylinder with the air pumped out - could show electrical conductivity and emit light. The color of light depends on the residual gas in the tube. In the discharge tube, Fig. 5, electrons are
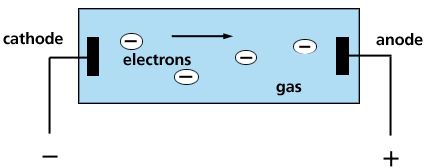
Figure 5. Gas Discharge Tube
emitted from the negatively charged cathode and move down the tube toward the anode. The electrons collide with the gas atoms and knock electrons out - creating positively charged gas ions. The electron-atom collisions rearrange the electron distributions in the energy levels and shift the electrons to higher energies. The atom is in an excited state and emits light as the electrons return back to the non-excited state.
The neon tube, developed about 1910 by Georges Claude, is basically a gas discharge lamp powered by a standard, household alternating current (AC) through a small transformer to produce 10,000 V AC. Neon gas gives a red color while argon (or argon plus mercury) gives blue. Other colors can be obtained using phosphor colors as in fluorescent lamps.
Return to the Readings Page
Go Back to the Previous Page
Page authored by the ACEPT W3 Group
Department of Physics and Astronomy, Arizona State University, Tempe, AZ 85287-1504
Copyright © 1995-2000 Arizona Board of Regents. All rights reserved.