Absorption of Light
The mechanism for the production of color by materials is the selective removal of certain wavelengths (or energies) of light from the electromagnetic spectrum of white light. The light must penetrate into the material and encounter energy-absorbing events for this selective removal to occur. A photon of given energy transfers all its energy to an electron in the absorbing material. The photon is "lost" from the light beam as it is absorbed, and the electron is excited to an available higher energy state by the gain in energy from the photon. The amount of absorption of photons depends on the energy levels available to the electron which absorbs the photon energy. A photon is not absorbed if there are no energy levels available for photon-induced electron transitions. The non-absorbed photons are scattered (or reflected) back, producing the sensation of color specific to the material.Pigment Response in the Infrared
In the infrared (IR) portion of the spectrum the wavelengths are greater than 700 nanometers with photon energies less than 1.8 electron-volts (eV). These infrared photon energies, typically around 1 eV, are so low that the photons are not absorbed by most pigments. The paint layers are therefore relatively transparent to infrared radiation. Infrared radiation penetrates the upper paint layers, but is absorbed by dark preliminary drawings made with charcoal that reside beneath them. The remaining radiation is reflected by white or light-colored grounds. With the aid of infrared sensitive photo equipment, the underdrawing can be seen because of the difference between absorbed and reflected radiation.A particularly convincing demonstration of the transparency of pigments to the infrared is provided in Fig. 6. In this case, (Fig. 6a) an underdrawing is made using charcoal on a white ground. A layer of paint (Fig. 6b) is then applied to the drawing which is viewed with an infrared sensitive video camera. The image, Fig. 6c, of the underdrawing can be seen clearly in the infrared display.



Figure 6. Demonstration of infrared reflectography. a) photograph of charcoal drawing in visible light, b) photograph of overlaid paint layers which hide the charcoal drawing, c) infrared reflectogram revealing the charcoal drawing beneath the paint layer.
Fluorescence with Ultraviolet Light
Photons of visible light are not absorbed in layers of varnish and binding media in painting but are transmitted through them. The higher energy photons in the ultraviolet (UV) region of the spectrum are strongly absorbed in these varnishes and binding media.The absorption of ultraviolet photons causes chemical reactions resulting, in some cases, in the emission of photns in the visible region of the spectrum. The absorption of light photns of high energy and re-emission of photons of lower energy, in the visible region, is commonly called fluorescence.
The fluorescence process sketched in Fig. 7 depicts an incident UV photon interacting with an electron. The photon is absorbed and transfers its energy to the electron raising it to one of the availabe energy levels. This enhanced energy of the electron may be partially lost by heat (process 2 in Fig. 7) and then thte electron can make a transition back to the original state by emitting a photon (process 3 in Fig. 7). The emitted photon is of a lower energy than the incident photon. Aged varnishes have different fluorescent properties than new varnishes and some modern pigments fluoresce differently than traditional pigments. Thus examination of a painting under ultraviolet light can often reveal retouched or restored areas.
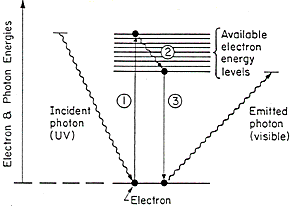
Figure 7. Illustration of the fluorescence process: An incident ultraviolet photon is absorbed and transfers its energy to an electron (process 1), the electron loses some of its energy in non-radiative processes, 2, and then the electron makes a transition to the ground state, process 3, emitting a photon with energy in the visible region.
Return to the Readings Page
Page authored by ACEPT W3 Group
Department of Physics and Astronomy, Arizona State University, Tempe, AZ 85287-1504
Copyright © 1995-2000 Arizona Board of Regents. All rights reserved.