in classical notation with m=mass of the electron; electrons are deflected by electric fields whereas the photon path is undeflected. The list of differences goes on.
Yet, both electron and photon are at the heart of modern quantum mechanics with both described by wave and particle behaviour. The wave behaviour is demonstrated by the similarity of electron and X-ray diffraction. The particle behaviour is illustrated by the photoelectric effect with photon incidence on a solid surface leading to electron ejection.
The electron is negatively charged and is deflected by an electric or magnetic field. It is this deflection that is at the basis of the operation of the modern television set or computer monitor. One hundred years ago, the electron deflection was observed directly in a cathode-ray tube (electrons were originally called "cathode-rays"). In a cathode-ray tube, Fig. 1, electrons
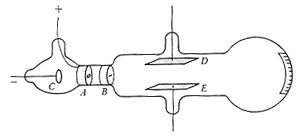
Figure 1. The cathode-ray tube. Electrons from the cathode C pass through the slits at A and B and strike a phosphorescent screen. The beam can be deflected by an electric field between the plates D and E or by a magnetic field (not shown).
are emitted from a heated (for electron emission) negative electrode (the cathode, C) and are attracted to the positively charged anode, A. They pass through a slit in the anode into the main part of the tube where they are deflected by the plates. If the upper plate is charged positively, the electron is deflected upward (opposite charges attract) and its motion is detected on the phosphorescent screen at the end of the tube.
The electron is one of the elementary particles, called leptons, which include muons and neutrinos among others. The most obvious feature of the electron is its electric charge, written as "e", which has a value of
where the Coulomb is equal to the charge transferred by a current of one Ampere in one second.
The electron also has mass where the mass is given by
a value which is about one-two thousandth the mass of the neutron or proton -- the particles that make up the positively charged nucleus of the atom. The ratio of the mass of the proton mp to that of the electron me is
The energy of the electron volt is given in units of electron volts with the symbol eV. One electron-volt is a unit of energy equal to the work done on an electron moving it through a potential difference of one volt.
Electrons occupy well-defined energy levels around the positively charged nucleus of an atom. The positive charge is determined by the number of protons and gives the atomic number, Z, of the atom. Vacancies in the inner electron levels of the atom can be produced by irradiation with X-rays or energetic electrons or protons. The excited atoms can release their energy by emission of photons such as X-rays or by emission of electrons. This last process, discovered by Pierre Auger in 1927, is known as Auger emission and is the dominant de-excitation process for low atomic number elements such as boron or carbon. Figure 2 illustrates the Auger process.
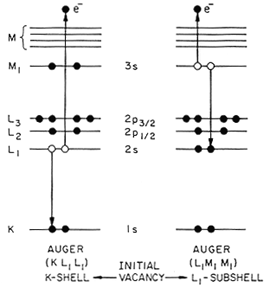
Figure 2. Schematic diagram of various two-electron deexcitation processes. The KL1L1 Auger transition corresponds to an initial K hole which is filled with an L1 electron and simultaneously the other L1 electron is ejected to the vacuum. The LM1M1 Auger transition is the corresponding process with an initial 2s vacancy.
Electrons and photons have wave-like behaviour. Of interest in comparison of optical and electron microscopy is the fact that energetic electrons have wavelengths orders of magnitude smaller than that of visible-energy photons. The resolving power of a microscope is determined by the wavelength of the probing particle. Hence a scanning electron microscope can "see" finer details than an optical microscope. This can be shown by the following calculation.
The kinetic energy of an electron of energy eV equals 1/2 mv2 where m is the mass and v is the velocity.
The momentum of the electron is mv
and the wavelength 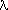 is equal to
is equal to
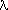 = h / momentum = h / (2eVm)1/2.
= h / momentum = h / (2eVm)1/2.where h is Planck's constant, h = 4.136 x 10-15 eV s. Then for an electron energy of 3600 eV (typically used in scanning electron microscopy) the wavelength of the electron beam is 0.02 nanometer, a value 10,000 times smaller than that of visible light used in optical microscopy.
Continue to the Electrons in Atoms Reading
Go to the Readings Page
Go Back to the Previous Page
Page authored by ACEPT W3 Group
Department of Physics and Astronomy, Arizona State University, Tempe, AZ 85287-1504
Copyright © 1995-2000 Arizona Board of Regents. All rights reserved.