|
Organic Structure Determination 2 |
Analytical Chemistry
|
|
|
|
Summary of Instrument-based methods for determination of structure of organic molecules�
� Mass Spectrometry - yields molecular weight/elements/possible molecular formulas
� Infrared Spectroscopy - yields functional groups
� NMR Spectroscopy - very important, yields structure
|
1 NMR Spectroscopy - Structure Determination |
|
1.1 Basic Principles: Nuclear Spin and Radio Frequency Absorption |
� All charged objects that move generate a magnetic moment, i.e. have the property of magnetism
� We can think of electrons (negative charge) and protons (positive charge) as spinning, when they spin they generate a magnetic moment, this magnetic property of these particles are described as their "spin"
� this spin is the information that is contained in the spin quantum number for electrons (+1/2 and -1/2)

� HOWEVER, this is classical picture of magnetism and spin, and spin is a quantum mechanical phenomenon, the classical picture misses some maybe confusing but important details, specifically:
1) only TWO spin states are possible for electrons and protons (the +1/2 and -1/2 quantum numbers), and�..
2) neutrons can also have spin (even though they have no charge, this is a quantum mechanical physics thing!)
� Taking the individual spins of the protons and neutrons into account and how many there are, then the nucleus as a whole CAN have an OVERALL SPIN, depending upon whether the neutron and protons cancel or not
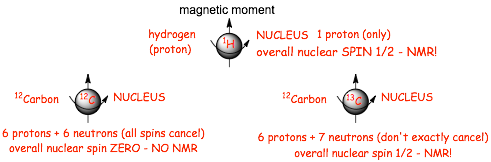
� For NMR to work, the nucleus must have an overall nuclear spin, this is the case for 1H hydrogen (the nucleus is a proton) and also the 13 isotope of carbon, but not the most abundant 12 isotope of carbon
� We can do NMR on nuclei of hydrogen atoms (single protons) and the nuclei of 13C atoms (and other atomic nuclei that we do not cover in this course)
� Overall nuclear spin can also have two states, we will call these alpha and beta, and when a sample containing 1H and 13C atoms is placed in a larger (external) magnet, these two magnetic spin states align either with the field (alpha) or against the field (beta).
� the alpha nuclear spins are now LOWER IN ENERGY and the beta nuclear spins are now HIGHER IN ENERGY in the large external magnet and its associated magnetic field

� NOW THAT THEY ARE IN THE MAGNET, there is an energy difference between the alpha and beta spins, and we can equate this energy difference to a specific frequency of electromagnetic radiation using the usual equation
� AND, the energy gap depends upon how large the magnetic field is, put the sample in a LARGE MAGNETIC (large H0) and the energy gap is LARGE, in a small magnetic field (small H0) the energy gap is small, in NO magnetic field, there is no energy difference between the alpha and beta spins
� Electromagnetic radiation energy can convert the lower energy alpha spins into higher energy beta spins

� the frequency of the radiation required to do this conversion alpha to beta is called the resonance frequency, it depends upon the energy gap (Delta E), and thus the magnetic field (H0), but even in very large magnetic fields the frequency is very small, and the frequencies are much lower than IR, or even microwave, they are in the radio range of the electromagnetic spectrum
� it is a resonance frequency because after an alpha spin is converted to beta, the beta will eventually find a way to return to the lower energy alpha spin state, releasing the energy (as a small amount of heat), where it absorbs radiation again to form beta, then to alpha etc., the radiation induces a resonance between the alpha and beta spin states
� Scanning through all of the possible frequencies for a sample in a magnetic field reveals the resonance frequency because the sample ABSORBS the radiation of this frequency (ONLY), it uses the energy to induce the spin change, and so absorption versus frequency is the NMR spectrum (simple version below)

|
1.2 Shielding and Deshielding |
� MOST IMPORTANTLY the frequency tells us the energy difference between the alpha and beta spins, Delta E
� AND, even though all of the nuclei in a molecules are in the same external magnetic field (H0), the LOCAL magnetic field at each individual nucleus in the molecule can be different due to electron SHIELDING
� The electrons around a nucleus generate their own field that opposes the external field, i.e. electron motion magnetically SHIELDS the nuclei
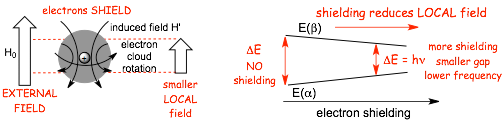
� NOW, it gets a little confusing, because ALL nuclei are shielding in molecules by the electrons, when the shielding is reduced, for example by reduced electron density at an atom, the nucleus is said to be DESHIELDED
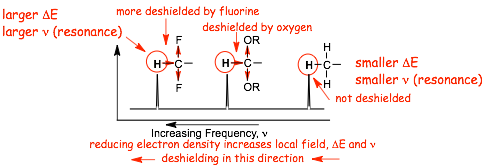
� DESHIELDING can occur when there is an electronegative atom close by, the electronegative atoms effectively "pull" electron density away from proximal hydrogens
� This DECREASES the nuclear shielding, INCREASES THE DESHIELDING, which INCREASES the LOCAL magnetic field, which requires a LARGER resonance frequency to convert the nuclear alpha and beta spins
Schematic EXAMPLE:
� NOT all nuclei are shielded to the same extent, this is not a problem this is the entire basis of NMR spectroscopy
|
1.3 Chemical Shifts |
� The differences in resonance frequency are actually very small, and plotting them in an absolute scale is not as useful as plotting them on a RELATIVE SCALE, this is the CHEMICAL SHIFT scale
� Chemical shifts are resonance frequencies RELATIVE to the signal from a standard, the usual standard is tetramethylsilane (TMS)
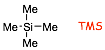
� chemical shifts are measured in the unit-less delta (it is a ratio), and range from ca. 0 - 10 ppm in proton NMR spectra and from ca. 0 - 200 ppm in 13C NMR spectra. The delta value for TMS is always zero, by definition.
|
1.4 Which Direction is Which in the Spectrum? |

� MORE DESHIELDING means LARGER LOCAL MAGNETIC FIELD, which means LARGE RESONANCE FREQUENCY, which means LARGER DELTA means LARGER CHEMICAL SHIFT
|
1.5 Which Groups Deshield and by How Much? |
� 2 main factors influence deshielding
(1) the inductive effect of substituents, i.e mainly electronegativity
(2) the magnetic effect of substituents, which is mainly a property of unsaturated groups.
� We are concerned with ELECTRONEGATIVITY, UNSATURATION and COMBINATIONS OF THESE TWO
Electronegativity. Let's look at a real spectra to illustrate.
Electronegative elements "pull" electron density from atoms via the INDUCTIVE EFFECT
(the nmr spectra included in these lecture notes were not run on an nmr spectrometer, instead they are simulated using a computer program obtained from: https://www.nmrdb.org/. This has the advantage that the spectra are very clean and easy for students to read, but the miss some realistic features such as noise, contributions from impurities, etc.)
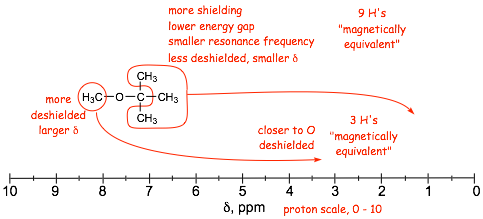
� Proximity to the electronegative oxygen results in reduced electron density due to the INDUCTIVE EFFECT, resulting in DESHIELDING, which increases the chemical shift, delta
� The farther away from the oxygen, the smaller the inductive effect, the lower the deshielding
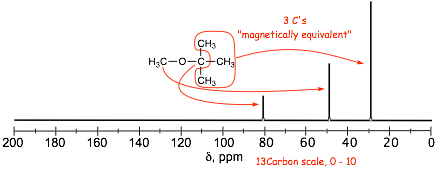
� Nuclei that experience the same LOCAL FIELD due to the same bonding and symmetry are MAGNETICALLY EQUIVALENT, they all contribute (add to) the same signal; in the NMR spectrum
� NOTE the similarity between the proton and 13C spectra. 13C spectrum gives number of equivalent carbons in the molecule
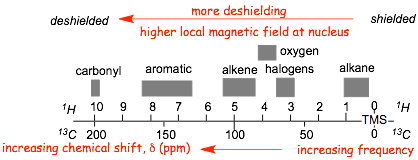
Unsaturation For example, the effect of proximity to pi bonds
� Electron movement induced by the external magnetic field in the pi-orbitals that contain the polarizable electrons is responsible for generating an ADDITIONAL local magnetic field which ADDS to the external field, effectively DESHIELDING the protons and carbons that are part of pi-systems
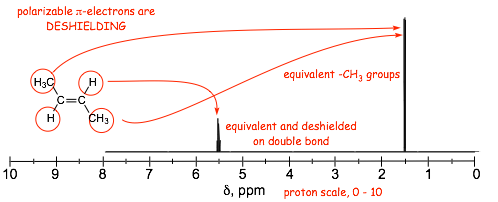
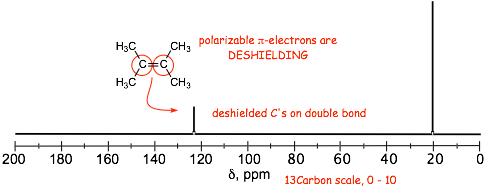
� deshielding by the benzene ring even larger than for alkene double bonds
� note the characteristic frequencies of the aromatic protons, i.e ca. 7 ppm, and 130 ppm.
� NOTE also that in the =example below, all 5 protons on the benzene ring have signals that are so close together they overall (even though they are not all magnetically equivalent, this happens sometimes)
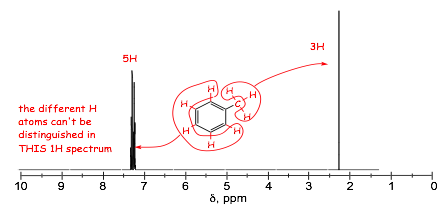
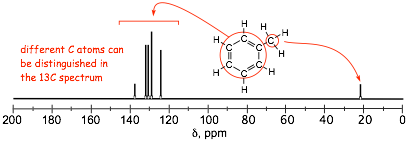
� in these spectra, the aromatic 1H signals overlap extensively and the magnetically inequivalent protons can not be distinguished, although they can in the 13C spectrum
Origin of the Deshielding (and Shielding) Effect of Unsaturation
� Deshielding by electronegative elements via the INDUCTIVE EFFECT is easy to understand, it is the electrons that do the shielding and so reducing electron density due to the inductive effect results on DESHIELDING
� Deshielding by unsaturation is different, in this case the movement of p-electrons in p-molecular orbitals generates local magnetic fields that result in decreased (or sometimes increased) overall magnetic fields at nuclei
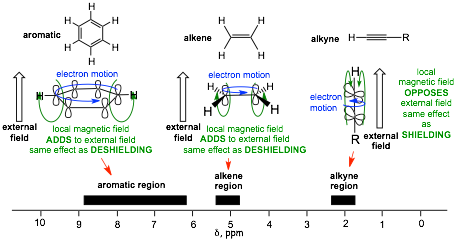
� The DIFFERENT SHAPES and ORIENTATIONS of the p-Molecular Orbitals result in DIFFERENT extents of DESHIELDING and in the case of alkynes, SHIELDING
Combined Unsaturation/Electronegativity For example, proximity to p-bonds AND electronegative oxygen
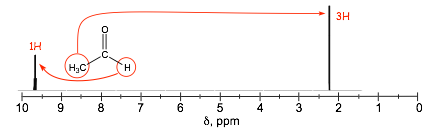

� unsaturation and electronegativity combine to give largest chemical shifts
� there are ranges of chemical shifts even for similar kinds of nuclei, but the ranges are fairly well defined.
This is all slightly tricky and is worth reviewing once more��

Finally, Extent of Substitution in Carbon Spectra
� 13C spectra are more sensitive to deshielding factors than proton spectra because there are more electrons around each C nucleus compared to H. Even the number of alkyl groups around a carbon can be determined in carbon spectra. Even though the electronegativity difference between C and H is small, replacing H with the slightly more electronegative C results in slightly more deshielding. Specifically, going from a primary carbon to secondary to a tertiary carbon results in signals that increasingly deshielded, let's revisit a spectrum from above....
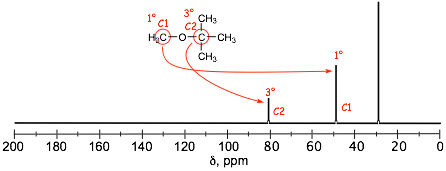
� Even though both of the circled C atoms are directly attached to oxygen, the "central" carbon of the t-butyl group, C2, is significantly more deshielded than the carbon of the methyl group C2 BECAUSE it is tertiary (has more C atoms attached to it) compared to C1, which is primary.
|
1.6 Signal Splitting: (N + 1) Rule and Coupling Constants |
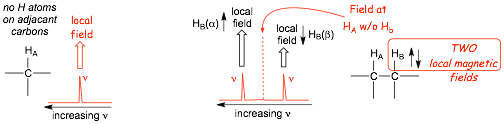
� In the absence of any other hydrogen atoms, Ha (ABOVE) has a conventional resonance frequency/chemical shift that is determined by its local magnetic field as usual
� the PRESENCE OF Hb, however, alters the local magnetic field at Ha, the TWO possible spin states at Hb results in both a small INCREASE in the local field (alpha spin) and also a small DECREASE in the local field (beta spin), this results in TWO NEW resonance frequencies/chemical shifts, the peak is split into TWO, it becomes a DOUBLET
� the presence of the TWO hydrogens HB (BELOW) result in THREE local magnetic fields at proton Ha, the peak for HA is similarly split into THREE, it appears as a triplet.
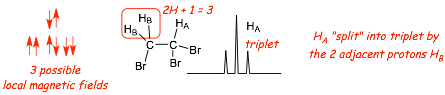
Splitting N+1 "Rule"
� N+1. The number of peaks into which a signal is split is equal to the number of non-magnetically equivalent protons with which it interacts + 1 (assumes all equal J value, which is explained below)
� in our courses, splitting only occurs with protons on ADJACENT carbon atoms, hydrogens on carbons further away usually have only7 a weak effect and can usually be ignored
� the area ratio's of the peaks in a splitting pattern is given by Pascal's triangle

� coupling constants (J) have to be identical for interacting protons (see below)
The coupling constant measures the frequency difference between the peak positions in a spilt peak, and is given the symbol J, which has units of frequency, usually Hertz, which is reciprocal seconds
� in the example below, the three H(a)'s "split" the two H(b)'s (and vica versa).
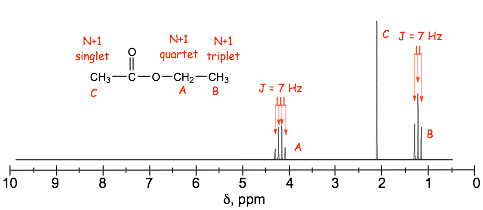
� the J coupling constants, the splitting, for both signals are identical because they are "coupled"
� J is usually ca. 7 Hz for protons on alkyl chains with free rotation
� The carbon nmr spectrum of the same molecule as in the proton spectrum above shows NO SPLITTING
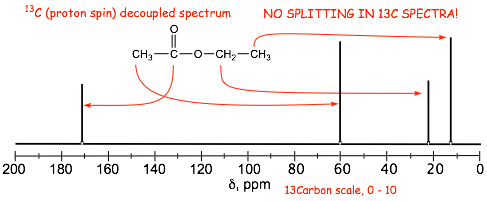
IMPORTANT!
1) All of the 13C spectra in OUR organic chemistry courses are "PROTON DECOUPLED", i.e. no splitting is observed in our carbon spectra peaks, only singlets are observed.
2) The same factors determine chemical shift in both the proton and carbon spectra.
3) There are often more peaks in the carbon spectrum compared to the proton spectrum, which means that one of the carbon atoms does not have any hydrogens attached (the C=O carbon in this case)
Example Problem: Assign the protons to the peaks in the provided spectra
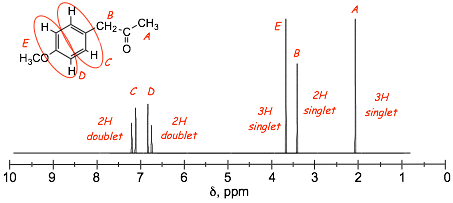
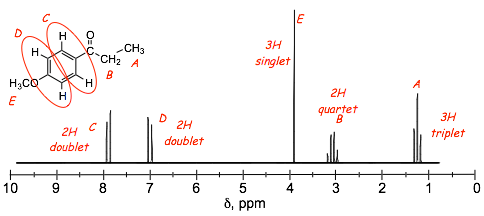
� how are the benzene protons C and D distinguished in these spectra?

� minor resonance contributors tell us about minor changes in electron distribution in the benzene ring
� a partial negative charge means increased electron density, more shielding, LESS DEshielding, smaller chemical shift, these are the protons indicated a D in the structures above
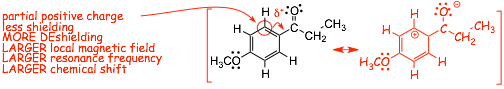
� a partial positive charge means decreased electron density, less shielding, MORE DEshielding, larger chemical shift, these are the protons indicated a C in the structures above
|
1.7 Signal Sizes: Integration |
� In 1H spectra, the signal size relates to the number of contributing protons
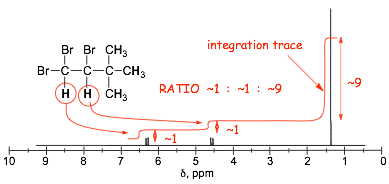
� NMR spectrometers provide relative signal sizes ONLY, historically as an integration trace, such as that shown superimposed on the spectrum above
� It is the INTEGRATED AREAS under the peaks that determine signal "size", not peak height
� signal size is related to # of equivalent H's in proton spectra
� in most of the spectra you will see, however, the ABSOLUTE number of hydrogens that contribute to a signal are given (which is a lot easier)
� signal size is NOT related to # of equivalent C's in 13C spectra due to spin relaxation effects
|
1.8 Complex Splitting |
� Breakdown of the (N + 1) rule occurs when protons are split with different coupling constants (J)
� Splitting by different protons with different J value results in complex splitting patterns, usually best understood with the aid of a "splitting tree" (note the "order" of the splitting does not matter)
� For the example of Ha�..
Ha is split by Hb with a coupling constant (J) of 15 Hz (not the usual 7 Hz)
Ha is ALSO split by Hc with a different coupling constant (J) of 10 Hz (again, not the usual 7 Hz)
the result is a DOUBLET OF DOUBLETS
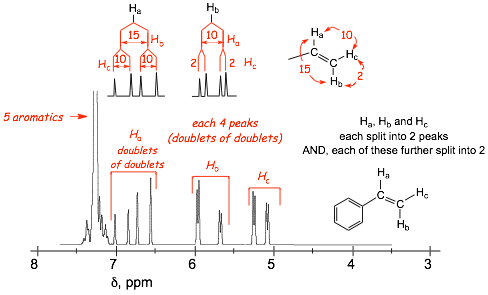
(note the expanded horizontal chemical; shift scale in this spectrum to show the small splittings)
� the protons Ha and Hc are also doublets-of-doublets, although one of the splittings is small
� the SPLITTING TREE shows these two splittings that result in the doublet of doublets
|
1.9 Exchangeable Hydrogens |
� under usual conditions, N-H and O-H protons exchange on the NMR timescale due to hydrogen bonding with each other and with small amounts of water and particularly acid or base impurities (it is quite difficult to completely dry most organic solvents

� in many of the nmr spectra the timescale of the hydrogen atom exchange is faster than the timescale of the nmr experiment, and "blurred" or averaged signals for O-H protons are observed
� the chemical shifts of O-H (and N-H) protons are thus very condition dependent (e.g. they vary with concentration, see below), AND, it is not really possible to use our usual understanding of deshielding to predict the chemical shifts of O-H and N-H signals!
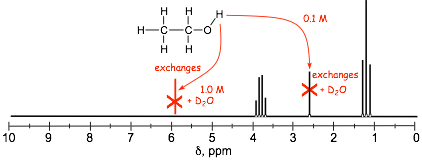
� Because of this "blurring" or averaging of exchanging protons, splitting is not observed (they are SINGLETS), they are not split and they do not split
� because they are exchangeable, O-H (and N-H) signals ALSO usually disappear upon shaking with D2O because the H is exchanged for D, and D is not visible in a proton nmr spectrum
� signals may or may NOT be indicated as exchangeable on a nmr spectrum, but if they are then this is very useful diagnostically, it shows that the protons are bonded or oxygen (or nitrogen)
|
1.10 Real Spectra |
� Real spectra are often not as simple as those provided in this lecture course!
� You are usually given the absolute number of hydrogens for each peak in 1H NMR, however, real integrations give only relative numbers of hydrogens, peak ratios have to be converted into absolute hydrogen atom count
� Spectra are run in solvents in which the H's have been exchanged for D's, however, exchange is never complete and peaks due to solvent are often observed

� In addition to peaks from the solvent, real spectra often contain peaks from impurities and other contaminants
� Small peaks, e.g. on tertiary carbons, may get "lost" in the noise in 13C NMR spectra
� the peak from the TMS is, of course, not part of the spectrum of the compound being studied, it is present in order to calibrate the chemical shift scale (TMS is often not observed in modern spectra)
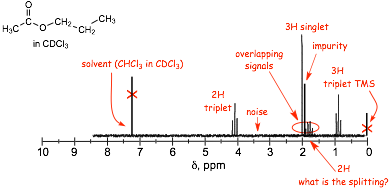
Example:
� Determining the exact splitting is difficult for peaks that are highly spilt, for example, what is the splitting pattern for the 2H signal in the spectrum above?
� In some cases it is only possible to say that a signal is split, without actually saying exactly how many peaks it is split into, such a peak is described as a MULTIPLET
� Finally, there are often cases where different signals overlap, again, as shown above.
|
2 Solving Spectral Problems; A Suggested Procedure |
� For most of the spectrum problems you will be asked to solve in this class you will be provided with the molecular formula: here is the suggested procedure for these problems
1. Get degrees of unsaturation from the molecular formula
2. Get functional group information from the IR
3. Get the number of chemically inequivalent carbons, and confirm functionalities from 13C NMR
4. Compare # of signals in proton and 13C NMR's to determine whether there are carbons without hydrogens
5. Build molecular fragments and put them together to make tentative structures
6. See if the structures fit the proton NMR. Important! Try to get as much information from the other spectra before going to the proton NMR
Example Problem 1: Determine the structure and assign the peaks in the proton NMR spectrum
Molecular formula = C6H12O2 1 degree of unsaturation
� this implies 1 double bond OR 1 ring but not both
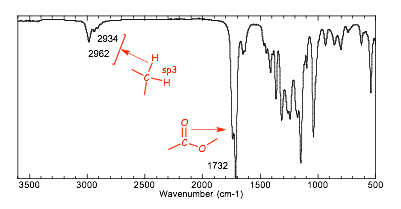
� The IR spectrum clearly shows no C=C unsaturation (no C-H stretch above 3000 cm-1), and clearly shows a carbonyl group. This is around 1740 cm-1, and so must be an ester, which means that we have "found" the one degree of unsaturation
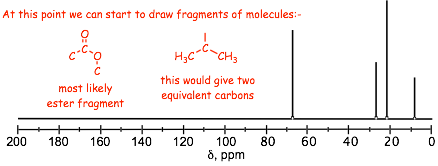
� the 13C NMR confirms the carbonyl carbon with high chemical shift
� the spectrum has only 4 additional peaks, which means that there must be 2 magnetically equivalent carbon atoms, this is commonly an isopropyl group
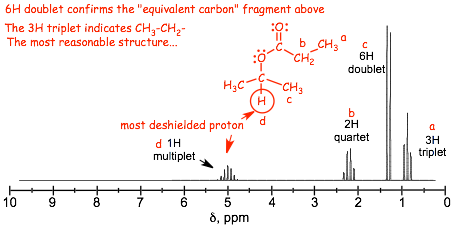
� look for the methyl groups in the proton NMR, they come in integral multiples of 3, they tend to be less deshielded because they are at the ends of chains, and from their splitting patterns you can figure out what they are connected to
Important! After you have your structure, predict a proton spectrum and see if it matches the provided spectrum. You should not be in any doubt as to whether you have the correct structure
Example Problem 2
Molecular formula = C8H10O 4 degrees of unsaturation
� We immediately think about a possible benzene ring (which has 4 degrees of unsaturation on its own)
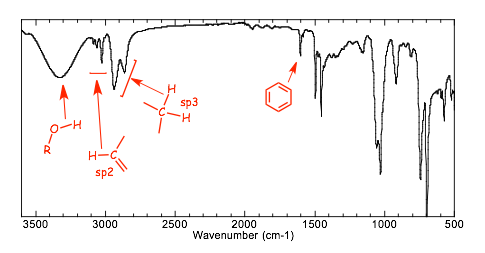
� The IR spectrum clearly shows an �OH group, and C=C unsaturation (C-H stretching vibrations above 3000 cm-1), and evidence for a benzene ring stretch at ca. 1600 cm-1
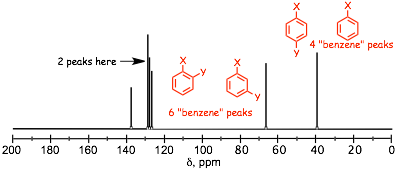
� the 13C nmr spectrum confirms the benzene ring, and there are TWO other peaks in "aliphatic" region that can not be magnetically equivalent
� there are 4 signals in the aromatic region, thus the substitution pattern on the benzene ring must be either a monosubstituted ring, or a 1,4 substituted ring
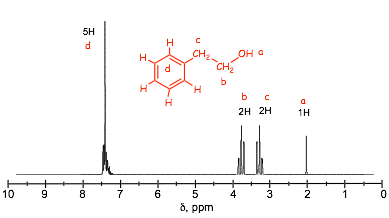
� 5 protons in the aromatic region conform that the benzene must be monosubstituted
� NOTE: in this case the aromatic region have a 5H peak that is split, this doesn't men that the 5 H atoms on the benzene are magnetically equivalent but that the chemical shift differences are small and that the signals OVERLAP, this is often observed when a benzene ring has a simple alkyl substituent
� the two 2H doublets tell us that there are no methyl groups (which would have a 3H signal) and that the structure has to be as shown, the more deshielded 2H signal must be the one attached to the electronegative oxygen
� REMEMBER: H atoms connected to O (or N) are exchangeable and are never split and do not split and have chemical shifts that are very variable and difficult to predict
Example Problem 3: Determine the structure and assign the peaks in the proton NMR spectrum
Molecular formula = C10H12O 5 degrees of unsaturation
� therefore we immediately think about there probably being a benzene ring
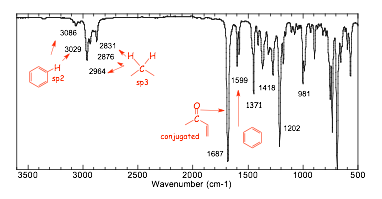
� the IR spectrum confirms the presence of a benzene ring and also suggests a conjugated C=O
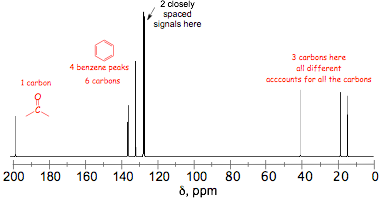
� the 13C NMR confirms the benzene ring (signals in the aromatic region) and the presence of a C=O (the highly deshielded signal) and 3 other carbons, none of which are magnetically equivalent
� the four signals in the aromatic region suggest either a mono- or a 1,4-disubstituted benzene
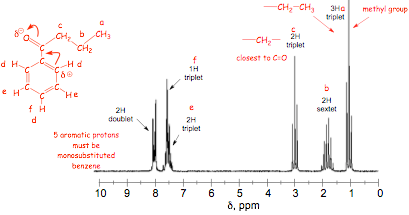
� 5 hydrogens are observed in the aromatic region, which means that the benzene must be mono-substituted
� the alkyl chain structure is obtained from analysis of the splitting patterns
� the aromatic signals are assigned on the basis of their integrations, splitting, and shielding
Important! After you have your structure, predict a proton spectrum and see if it matches the provided spectrum. You should not be in any doubt as to whether you have the correct structure