What is wrong with this picture?
I must admit that the connection of this Real Life page to resonance is a bit "loose"

This may be a bit out of order, but we only really understand all of the consequences of resonance and the chemical reactions of systems that exhibit resonance by via a detailed description of the wavefunctions of the extended molecular orbitals. This goes beyond the molecular orbitals that we discussed in the previous section of the notes, and we won't cover this properly until the second semester course. However, because we have just looked at molecular orbitals in the previous section, and because we will return to them later for a deeper explanation of resonance, it is worth looking at orbitals, particularly the hard part, The wavefunctions, i.e. the quantum mechanical part!
TAt the top of this page is the standard "picture" of an atom that you see everywhere, even on the web pages of University science departments (not ours!), but it is obviously incorrect. We all know that the electrons do not orbit around the nucleus like planets around the sun. We also know that it is not even possible to say exactly where an electron is in an orbital, never mind describe its exact path. The energies and locations of electrons in orbitals are determined by the laws of electrostatics and Quantum Mechanics.
Quantum mechanics describes atomic and molecular orbitals in terms of wavefunctions. Are you happy with this description of the orbitals, does quantum mechanics make sense to you? Hard to accept, isn't it! Quantum mechanics is hard to understand. Niels Bohr, a leader in early Quantum theory, once remarked that anyone who is not shocked by quantum mechanics hasn't understood it! Quantum theory can only be completely understood mathematically, and the math is harder than just about all of you have ever been exposed to. Richard Feynman once said "I think I can safely say that nobody understands quantum mechanics." So, how are we supposed to?
We obviously can't explain all of quantum mechanics here, but here is just a little more information. If you want to know what quantum mechanics has to do with organic chemistry, then we can't understand orbitals and why there are orbitals without it. If you want to know what quantum mechanics has to do with real life, then my answer is just about everything!
Quantum mechanics shakes the foundations of the world reality that you know! For example, it says that Newtons laws aren't (exactly) correct. According to the Newtonian view, properties can be measured (with precision). Newton says that if you want to, you can measure the momentum of a moving baseball, with any arbitrary precision, quantum mechanics says you can't. Newton would say that you should be able to determine exactly the position of an electron in an atom, quantum mechanics says you can't, which is why we only give the probablity of finding an electron at a certain distance from the nucleus. Newton says that if you want to, you can give a baseball any amount of (kinetic) energy depending upon how hard you throw it. Quantum mechanics says you can't. Newton would say that an electron in an atom can have many energies, depending upon the way that it "orbits" the nucleus. Quantum mechanics says it can't, the electrons can only have discrete energies given by the energies of the 1s, 2s, 2p etc orbitals. None of this is Newton's fault, he wasn't able to make observations on an atomic scale,and if he had then Newton;slaws might be different.
So what exactly is quantum mechanics saying? One thing is that the idea of precisely measuring properties is not possible, there is an "uncertainty" associated with measurements. This "uncertainty" has some strange consequences, for example the (perhaps small) probability that the particle will be somewhere it has no right to be, from the point of view of classical Newtonian physics. Apart from the fact that your understanding of the physical world around you is flawed if you take only the Newtonian view, some people extend this behavior of the physical universe to many diverse fields such as literary deconstruction (that argues that absolute meaning is always deferred, pushed back on something else, and therefore is never defined and thus never present) and even New Age philosophies that deny the presence of absolutes. I once had a long conversation with a church minister who wanted to know some basic principles of quantum mechanics. She was very interested about this fundamental idea of the lack of absolutes. If you think about it, it is pretty significant, huh?
Obviously Newton's laws work well enough for baseballs, and quantum effects only become important for really small systems such as atoms, but the philosophical principle is the same at all length scales! So, now you are convinced of the importance of Quantum Mechanics and you want to learn more. How to do it? Unfortunately, I find that many of the popular science books that address the subject (e.g. Stephen Hawking's books) to be very unsatisfactory. They will say things like "a particle is associated with a wave". OK, I understand the English words, but what does that really mean? One book that I do think is pretty good was written by a physicist who was (somewhat) involved in the development of the theories, Thirty Years That Shook Physics: The Story of Quantum Thoeory, by George Gamow. Quantum concepts are difficult to comprehend. I found that one way that worked for me was to to be exposed to ideas from several different directions and to slowly build up a "library" of examples of physical phenomena that could only be explained using quantum concepts. Eventually the weight of the evidence begins to overwhelm the ingrained sense of opposition to the ideas, and you slowly become a quantum believer! The only way most people will be able to do this is to take a physical chemistry or physics quantum course.
So, to take a baby step in the direction of understanding why quantum mechanics has to be be the way that it is, let's start with the most basic principle, i.e. the quantum. The word quantum is derived from Latin for "amount". Here is the basic idea. According to Newton, an object can have any arbitrary amount of energy. Quantum mechanics says an object can only have certain quantities of energy, i.e. quanta of energy. An object may have one quantum of a particular kind of energy, or two, or three etc, but never 1.5 etc. So, how big is this quantum? Well, it depends upon what kind of energy and what you are worrying about the energy of.
The concept of the quantum comes DIRECTLY AND NECESSARILY from the fact that particles have wave properties. Once you believe that particles have wave properties then you understand that it is impossible for them NOT to have quantized (non-continuous) energies (under the appropriate conditions). Actually, you already know of an example of a system with wave properties that has quantized energies. Pluck a violin/guitar string and it vibrates with a frequency (you get only one note) determined by its length, mass and how tightly it is strung. If you pluck it harder it vibrates with larger amplitude but the vibration frequency doesn't increase (the note doesn't change in tone), since only one value is "allowed" for this "plucking" strength and length/mass/tightness of stringing. The vibration of the string is CONFINED to act as a wave. If you pluck even harder, eventually a new (higher) frequency note is heard, the second harmonic of the first. With this new plucking strength, now two frequencies (notes) are allowed, but ONLY TWO. This is a direct consequence of the wave nature of the vibration, AND the boundary condition imposed by the length of the string etc. The vibrational frequencies of the string are "quantized", because the vibration is described by wave behavior AND there are boundary contains. The combination of these two means that only discrete, quantized vibrations are allowed, i.e. the standing waves. But you had to add the correct amount of additional energy by plucking to "populate" the second vibrational state (a bit of quantum language there!). You can't add half the energy required to get the second harmonic and get a frequency half as large, it is all or nothing. To populate the harmonic you had to supply enough energy to do so, you can't supply part of the energy and get part of a harmonic. This is why the vibrations of the string are quantized, only certain values are allowed BECAUSE the string vibration has the properties of a wave, the two are necessarily connected. This is illustrated in the picture below.
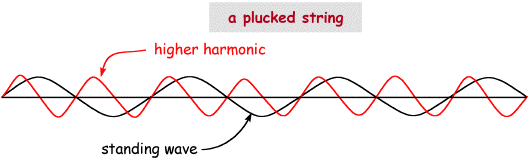
The same situation applies to electrons. Because they have the properties of a wave,with the appropriate boundary condition only certain waves will be allowed. These are the orbitals, and without taking the wave nature of the electron into account I have no idea how you would ever explain what there are orbitals! Although NOT accurate, a model that sometimes helps is to think of the electron actually moving with a wavelike motion. Like all pictorial descriptions of quantum effects this is just wrong since electrons really don't move like this at all. However, it is a way of illustrating the underlying concept behind orbitals having differing energies and sizes as a consequence of different wave properties. Consider the red wave below and imagine that it corresponds to a particular atomic orbital of the grey atom. The "size" of the orbital matches the "wavelength" in the red case (wl), a standing wave is setup and a valid "orbital" exists for this situation, characterized by the wavelength wl, corresponding to the particular energy associated with wl. The boundary conditions in this case that determine what the allowed wavelengths are include the charge on the nucleus and the electron, the kinetic energy of the electron etc. Only specific standing waves are allowed, these are the orbitals.

Finally, imagine an electron in a 2s orbital on, for example, atomic lithium. Give the electron some energy (for example by shining light on it). If the light energy is equal to the energy difference between the 2s and 2p orbitals then the electron can be promoted into the 2p atomic orbital (the 2p A.O. can be "populated" with the electron). Remember that the orbitals are always "there" even if they have no electrons in them. If the light energy is less than the 2s->2p energy gap, then nothing happens, the electron can't go half way, this would not be an allowed energy for the electron.
A final note. Max Planck, another Quantum pioneer, was also a philosopher of science. In his Scientific Autobiography and Other Papers, he gives "Planck's Principle", i.e. "A new scientific truth does not triumph by convincing its opponents and making them see the light, but rather because its opponents eventually die and a new generation grows up that is familiar with it." :\