|
Radical Reactions |
Non Lewis Acid/Base Mechanisms
|
|
|
|
|
1 Photochlorination and Photobromination |
� Most reactions occur via Lewis acid/base mechanisms and involve HETEROLYTIC cleavage of bonds and IONIC intermediates
� Under some circumstances, however, reactions can occur that involve HOMOLYTIC CLEAVAGE of bonds and RADICAL intermediates
|
1.1 Photochlorination of propane (as an example) |
� Photochlorination: "Photo-" means using light, specifically light (at an appropriate wavelength)
� Chlorination: means adding chlorine to a structure, except that this is VERY sloppy language since the -Cl must replace something, usually -H, in a SUBSTITUTION reaction
The reaction
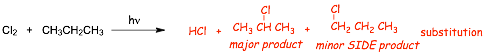
� Note: the use of the notation hv = light (energy), compares to D = heat (thermal energy)
the "v" in hv is actually the Greek "nu", the Greek version of n that looks like a "curvy" v, and so we often just use the lowercase letter "v" in hv
The mechanism - for formation of the MAJOR product
� Immediately we will see a difference compared to conventional Lewis acid/base mechanisms of, for example, alkene addition reactions, the steps are not drawn linearly, step-by-step, instead they are separated individually
� at first sight this may appear confusing, but there are reasons for this, most importantly the mechanisms are usually not linear, there are "branches", and, it is often useful to separate the steps to discuss them individually
� Note: all bond cleavages are HOMOLYTIC, and are described in terms of bond dissociation energies
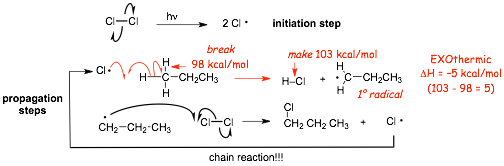
first step: the INITIATION step

� we are about to see that this mechanism is a CHAIN mechanism, the first (initiation) step INITIATES the chain
� the light energy (hv) supplies the energy required >55 kcal/mol to homolytically break the Cl-Cl bond
next steps: the PROPAGATION steps
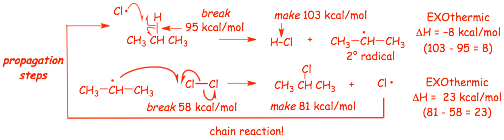
� the first propagation step breaks a C-H bond and makes a stronger H-Cl bond, the energy of the electrons goes DOWN, this step is exothermic, the exothermicity is determined by the difference in the relevant BDEs
� the second propagation step breaks a Cl-Cl bond and makes a stronger C-Cl bond, the energy of the electrons goes DOWN, this step is exothermic, the exothermicity is determined by the difference in the relevant BDEs
� the second propagation step forms a Cl atom, THAT REPEATS THE FIRST OF THE PROPAGATION steps, and continues in a CHAIN REACTION
� the chain reaction is not a linear sequence of reaction steps, which is why we write these mechanisms this way
common termination steps
� the CHAIN REACTION continues until one of the radicals or atoms in the propagation steps does something else, in which case the chain is then broken, and with continued light irradiation, another chain starts
� ONE termination step that breaks the chain is�.
![]()
� although this reaction does form the product, it is NOT INCLUDED as part of the conventional mechanism, because it is NOT A PART of the chain reaction and it is MUCH LESS PROBABLE than the propagation steps that form the product because the concentration of the Cl atom and the propyl radical are so low that the probability of them colliding as shown here is rather low
� other common termination steps are��.

� the "wall" is the reaction container wall, atoms and radicals are so reactive that if they collide with the container wall they may form a bond to one of the atoms at the container wall, such reactions are hard to describe using curved-arrow pushing since it is not clear which atoms of the container the radical/atom may react with
IMPORTANT POINT: An initiation step is NOT REQUIRED for every reaction!
� Initiation steps are actually quite rare, for every propagation step there is much less than one initiation step.
� The reaction is initiated with light, and the light intensity that is used is usually quite low, and so at any one time there are only a very few reactions being initiated.
� HOWEVER, once the reaction is initiated, it can keep going with requiring another initiation because the Br atom that is formed in the second propagation step goes and starts another first propagation step in a chain reaction
� And so reaction can keep going without any initiation, until the chain terminates
� IN FACT, if there were one initiation step for every propagation step there would be a huge buildup of Br atoms that would recombine, and then termination would then be a major step. In practice it is hard to get a light source that its intense enough to do this, and you don�t need an intense light source anyway, because of the chain
When you shine light on a mixture of alkane and halogen (Cl2 or Br2), it is the halogens that will actually absorb the light energy, the halogens are slightly colored (Cl2 is greenish and Br2 is brownish), whereas alkanes have no color at all, this is why the first thing that happens if the light causing homolysis of the X-X bond, nothing can happen until bond homolysis.
Now, the halogen atom is surrounded by millions and millions of molecules, and, the light intensity is not high, which means that at any one time hardly any of the halogens have actually absorbed light and split into atoms, and so the concentration of halogen atoms will be very low. Also, the halogen atoms are very reactive and they react almost as soon as they form, and so they never build up any significant concentration compared to the concentration of alkane molecules.
Because the concentration of the halogen atoms is very small, it is much more likely that the halogen atom will react with an alkane rather than recombine with another halogen atom, and this starts the chain reaction going. Of course, recombination of 2 halogen atoms could occur sometimes, and this will be a chain-terminating step, but each halogen atom is more likely to react with an alkane.
Also the alkyl radical is not then attacked by a halogen atom, because again, the concentration of halogen atoms is much smaller than the concentration of unreacted halogens, and so the radical reacts with a halogen MOLECULE (not atom) to form the product and another halogen atom, which then starts the cycle again.
What Determines Which is the Major and Which Minor Product?
Mechanism for formation of the MINOR SIDE product
� termination steps are not shown, but are similar to those shown in the previous mechanism
� the step that is DIFFERENT compared to the mechanism for the major product is the FIRST propagation step, that in this case forms a PRIMARY (1�) radical
NOW, let's compare the enthalpies of the two steps that are DIFFERENT between the two mechanisms
� the faster of these two will determine the major product of the reaction
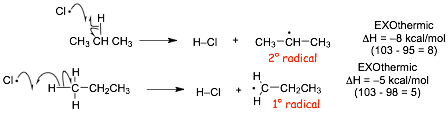
� According to the HAMMOND POSTULATE
� reaction to make the PRIMARY radical (that forms the MINOR SIDE PRODUCT) is less exothermic, and therefore should have a higher Ea, a later transition state and should be slower
� reaction to make the PRIMARY radical (that forms the MINOR PRODUCT) is less exothermic, and therefore should have a higher Ea, a later transition state and should be slower
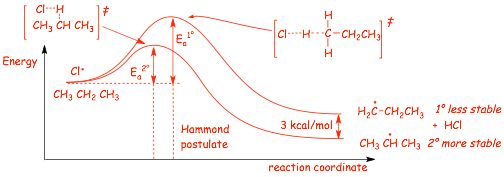
� The reaction that forms the MAJOR product has the FASTER critical step in the mechanism, although his step is exothermic for both reactions, both are fast and the major product isn't "very" major
|
1.2 Photochlorination versus Photobromination |
� Compare the products of photochlorination and photobromination of propane
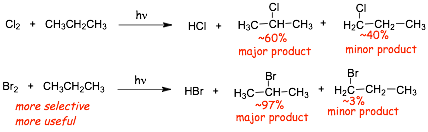
� Why is bromination MUCH more "selective" (and thus much more useful!) than chlorination?
� Compare the enthalpies of the steps in the mechanism that for the radicals that determine the major and minor products
� In photobromination H-Br is formed, the H-Br bond (88 kcal/mol) is WEAKER than the H-Cl bond (103 kcal/mol) which changes the thermodynamics of these two processes
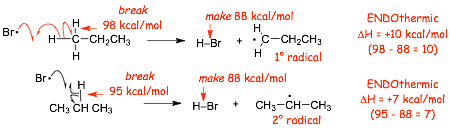
� BOTH reaction steps are now ENDOTHERMIC, but the reaction to form the 2� radical is LESS ENDOTHERMIC
� The Hammond postulate predicts a LARGER DIFFERENCE in activation energies when the reactions are ENDOTHERMIC, larger difference in rates results in INCREASED SELECTIVITY
� This is often called The Reactivity/Selectivity Principle
The reactivity/selectivity principle really only applies well to fast reactions such as those of chlorine and bromine atoms
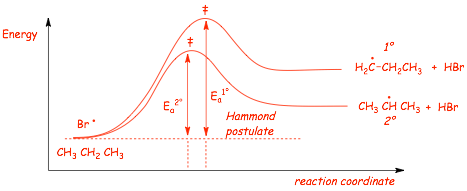
� There is a LARGER difference in Ea when the reactions are ENDOTHERMIC, which results in a larger difference in rate for the endothermic reactions, resulting in higher selectivity (reactivity/selectivity)
|
1.3 Photochemical Bromination is a Useful Reaction |
� What is the major product of the following reaction
� NOTE that organic chemical equations are often not fully balanced the emphasis is on the organic part of the reaction), AND, usually only the major organic product is shown unless there is a particular reason to show other products

� How to we explain the formation of THIS major organic product, i.e., why substitution of the particular hydrogen atom indicated instead of one of the other 11 H atoms in the reactant structure?
� We COULD do the entire mechanism, and if we don't make a mistake, the MECHANISM is our ALGORITHM for arriving at the correct product
� HOWEVER, by now we know that the critical intermediate in the mechanism is the radical that is formed by hydrogen atom abstraction by the bromine atom
� We can use HEURISTICS to "jump" to the critical part of the reaction, by only looking at all of the possible radical intermediates that could be made in this reaction:
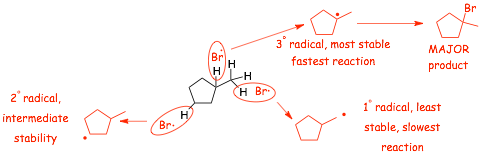
� the TERTIARY radical is the most stable radical we can make by doing a hydrogen atom abstraction reaction, this radical will be formed FASTEST, this radical gives the observed major organic product
Example problems: give the major organic products of the following reactions:
� EACH reaction proceeds via the most stable radical intermediate (shown in parenthesis), since that is the intermediate that will be formed FASTEST (the reaction is KINETICALLY CONTROLLED)
� Use HEURISTICS to quickly determine the major organic product by identifying the most stable radical intermediate
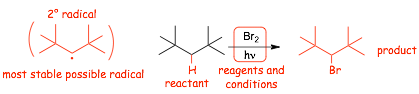

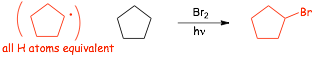
� note however, that in practice this reaction is only really useful if there is an obvious radical site that is more stable that any others, otherwise there will be multiple products (see later)
|
2 Allylic Bromination |
� THIS REACTION should work, and it does�..
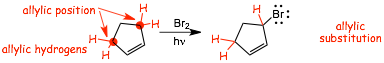
� this is substitution again of ONE of the four (in this case) allylic hydrogen atoms for -Br (if the reaction were run for longer times then more than one substitution would probably occur, care needs to be taken to properly control the reaction time and conditions)
� the reaction proceeds via the most stable radical intermediate, a resonance stabilized radical, as illustrated in the mechanism
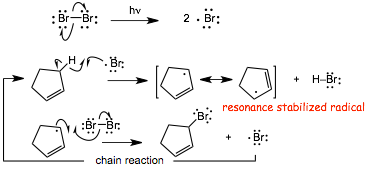
Wait a minute, didn't we just learn that Br2 ADDS to C=C bonds?

� Br2 DOES add to C=C bonds, and it is difficult to ensure that substitution occurs without any addition
� This problem can be SOLVED by using a VERY LOW concentration of Br2 to minimize addition to the alkene
This Requires a New reagent!

� Using NBS, low concentrations of Br2 are generated that can be photolyzed as usual to initiate the radical substitution reaction
� The mechanism for formation of Br2 from NBS is a bit obscure and uses H-Br that is often present as an IMPURITY, for this reason we will not go into the mechanism of the NBS reaction
Examples of Allylic Bromination
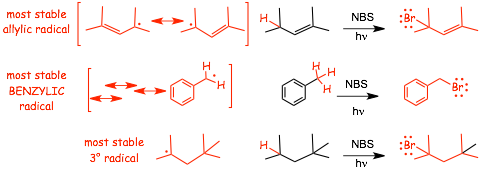
Some heuristics that you should expect to develop for these reactions:
1) The reaction is substitution of -H for -Br and proceeds via a resonance stabilized RADICAL intermediate and therefore there will be no rearrangements
2) NBS and light should be used instead of Br2/light to avoid direct addition of Br2 to the C=C bond
� You can USE NBS/light to do CONVENTIONAL bromination of a simple alkane also
� Use NBS and light as the reagents for allylic bromination, not Br2 and light
� Use EITHER NBS/light OR Br2/light for bromination of an alkane - MOST STUDENTS WILL USE NBS/light for ALL radical brominations (radical substitutions) to avoid making mistakes with the choice of best reagents
|
3 Anti-Markovnikov Addition of H-Br to an Alkene |
� In the previous section we learned how to do Markovnikov addition of H-Br to a C=C double bond in an ionic Lewis acid/base mechanism

� But what if we need the -Br atom on the LEAST substituted carbon of the C=C bond? i.e., what if we want to do ANTI-Markovnikov addition of H-Br to the alkene?
� This can be done using a PEROXIDE as an addition REAGENT in a RADICAL MECHANISM
� Light turns out to be not the best way of initiating this reaction, because unlike Cl2 and Br2, which are (slightly) colored and can interact with light, most alkenes and H-Br do not have any color, we need ANOTHER WAY of generating radicals
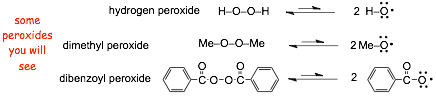
New reagent

� the oxygen-oxygen bond is weak due to electron repulsion between the non-bonding pairs on the adjacent oxygen atoms, and even just with a small amount of thermal energy it is possible to break the bond by homolysis to generate two alkoxy radicals that can initiate a RADICAL CHAIN MECHANISM
the O-O bond is also weak because the two oxygen atoms are smallish, which reduces the atomic orbital overlap, but that is beyond the scope if most general organic chemistry courses
� Unfortunately, there are several peroxides that can be used ion this reaction, either a specific peroxide may be specified in the reaction or just a generic "peroxides" may be specified, you need to recognize these various ways of specifying peroxides and how they are used
The Mechanism:
� As usual, radical mechanisms are not shown linearly step-by-step because they are often non-linear, in this case we have a non-linear CHAIN mechanism again
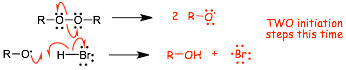
� There are TWO initiation steps this time
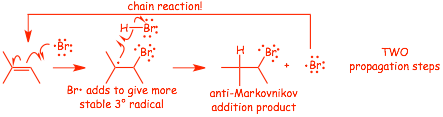
� There are TWO propagation steps
� And the usual termination steps, the following are examples (there are others, such as reaction with the wall)
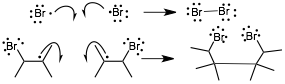
An Example Reaction
Compare the conventional Lewis acid/base reaction of an alkene with H-Br to the RADICAL REACTION that is initiated in the presence of peroxides
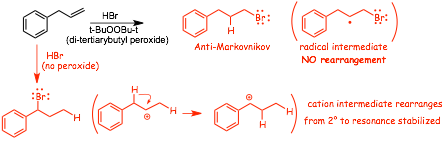
� the presence of PEROXIDES makes the RADICAL REACTION much faster than the Lewis acid/base reaction
� the reaction is REGIOSPECIFIC, the -Br adds to the LEAST substituted "end" of the C=C bond, ANTI-MARKOVNIKOV
� RADICALS DO NOT REARRANGE the same way that cations do, and so the addition is simple ANTI-Markovnikov (no rearrangements)
Example Reaction
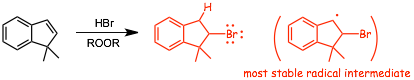
� This reaction is NOT STEREOSPECIFIC, because addition of a H atom to the intermediate carbon-centered radical can occur either from the "top" or the "bottom" equally, which means that the -H and -Br will be added both to the same side and also opposite sides, when stereoisomers can be formed (as in the case above), all isomers will be formed, which is why the structure above is shown with PLAIN bonds to both -Br and -H, it is not appropriate to use wedged/dashed bonds.
Some heuristics that you should expect to develop for this reactions:
1) These is an ADDITION reaction of -H and -Br across the C=C bond
2) This reactions is REGIOSPECIFIC ANTI-Markovnikov addition (-Br adds to most substituted end of the C=C bond where appropriate)
3) These reactions proceed via a RADICAL intermediate and therefore there will be no rearrangements
4) These reactions are NOT STEREOSPECIFIC, if cis/trans isomers can form they will form
|
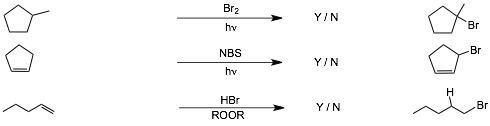
4 Reaction Summary |
Do NOT start studying by trying to memorize the reactions here!
Work as many problems as you can, with this list of reactions in front of you if necessary, so that you can get through as many problems as you can without getting stuck on eth reagents/conditions, and so that you can learn and practice solving reaction problems. Use this list AFTER you have worked all of the problems, and just before an exam. By then you will have learned a lot of the reagents/conditions just by using them and you will only have to memorize what you haven't learned yet. Then do the following:
� Cover the entire page of reagents/conditions with a long vertical strip of paper, see if you can write down the reagents/conditions for each reaction, check to see which you get correct, if COMPLETELY correct, circle Y, if incorrect or even slightly incorrect, circle N. In this way you keep track of what you know and what you don't know.
� Keep coming back to this list and so the same thing only for those reactions you circled N, until all are circled Y.
� Knowing the reagents/conditions on this page is INSUFFICIENT to do well on an exam since you will ALSO need to recognize how to use and solve reaction problems in different contexts, this page ONLY helps you to learn the reagents/conditions that you have not YET learned by working problems.