Organic Chemistry in "Real Life"
One of the most important uses of alkyl halides is in the form of freons as refrigerants.
Freons are chlorofluorocarbons. A definition would be a compound containing carbon and fluorine and also usually chlorine. Almost all compounds called freon contain only one or two carbon atoms and no hydrogens. They are non-toxic and non-flammable, and are thus good for use in household appliances/automobiles etc. Freons were invented in the 1920's after several accidents in refrigerators containing older more toxic refrigerants, notably ammonia and methyl chloride and sulfur dioxide. In 1930, Thomas Midgley, who worked for General Motors and headed the research group that invented freon, famously held a demonstration of the physical properties of Freon for the American Chemical Society by inhaling a lung-full of the new wonder gas and breathing it out onto a candle flame, which was extinguished. This demonstrated the gas's non-toxicity and non-flammable properties. Interestingly, Midgley also invented leaded gasoline! The poor guy didn't realize that he was responsible for two of the most damaging substances to be expelled into the atmosphere! The term freon at the time referred to a complex mixture of individual freon structures.
Of course, it now seems pretty clear that the presence of freons is damaging to ozone in the atmosphere. This is what happens:
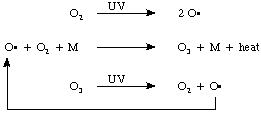
A lot of UV radiation hits the upper atmosphere. The UV light (photons) causes breaking (lysis) of the oxygen-oxygen bonds in molecular oxygen (photo-lysis!) to form oxygen atoms. This is basically the same process in which light is used to photolyze Br2 to give 2 bromine atoms we talked about in class. In the presence of a microscopic particle of dust, or some other substance, M, the oxygen atoms combine with oxygen molecules to generate ozone, and importantly, heat. The ozone is also photolyzed by UV light to regenerate oxygen and an oxygen atom, and heat. The oxygen atoms react with oxygen to make ozone and a cyclic process occurs whereby the energy of the UV light is converted into heat, and the harmful UV is prevented from reaching the ground.

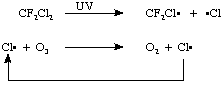
In the upper atmosphere, freons (an example is so-called freon-12, CF2Cl2) are also photolyzed by UV light. The chlorine atoms that are formed react with ozone to form a ClO radical and oxygen. This radical reacts with an oxygen atom to form molecular oxygen and another chlorine atom. This chlorine reacts with another ozone in a chain reaction, again, very much like the free radical chlorination reaction we studied in class. In this way many ozone molecules can be irreversibly destroyed!
The Nobel prize in chemistry 1995 was awarded to Paul Crutzen, Mario Molina and Rowland for work in this area. Since 1978, the use of freons in aerosol cans has been banned in the United States. In 1985, a hole in the ozone layer over the Arctic Circle was discovered. Studies suggest that this is due to freon photolysis, although I don't know if this has been confirmed with certainty yet.
The ozone depletion problem initiated the 1987 Montreal Protocol. This treaty required the signature nations to reduce production of chlorofluorocarbons (CFC's), of which freons are the obvious examples, and to date over 120 nations have signed on. Many industrial nations have now eliminated production of CFC's entirely.
The new "safer" alternative refrigerants contain hydrogen in addition to fluorine and carbon. Although they still contain C-Cl bonds, these compounds decompose before they reach the stratosphere and are thus less harmful.