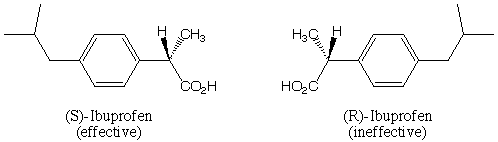
Organic Chemistry in "Real Life"
This week we return to the subject of steroisomers. In particular we will discuss a particular form of steoisomerism known as enantiomerism. You know that stereoisomers differ not in the connectivity of the atoms, but only by the different orientation of the atoms in space. Enantiomerism represents a particularly subtle form of this phenomenon. Two different enantiomers differ only in that one is the mirror image of the other. If you like, the molecules are identical except that one is "left-handed" and the other "right-handed". This difference seems to be very minor, and surely can't be significant can it? It turns out that it can be extremely significant.
Most of the naturally occuring organic compounds and structures in your
body have this property of left and right "handedness", including
DNA and in particular, the enzymes. A "left-handed" enzyme will
only react with a "left-handed" substrate, the "right-handed"
substrate will be ignored. This has some relatively benign consequencies.
A classic example that is of
Carvone. It has two "handed" forms, which are distinguished as
(R)-Carvone and (S)-Carvone (we will define (R)- and (S)- in class). The
(R)- form smells of spearmint and the (S)-form smells of caraway seeds.
Obviously our "smell" receptors are capable of making this apparently
subtle distinction. Another classic example that teachers often use is the
pain killer ibuprofen, whose structure is shown below. The drug is sold
as a 50:50 mixture (this mixture has a special name that we will learn)
of the two diffeerent "handed" forms R- and S- because it is easiest
to manufacture that way. However, only the (S)-form of the compound actually
acts as a pain killer, the (R)-form is completely ineffective. So, half
of the ibuprofen that you buy appears to be useless! This isn't quite correct
however, since in the body some of the (S)-form can be converted into the
(R)-form. These effects of enantiomerism are interesting, but not particularly
worrisome.
However, there is one further classic, and unfortunately extremely
tragic example that instructors often to illustrate the effects of enantiomerism
that is relevant to people born in Europe around the time that I was.
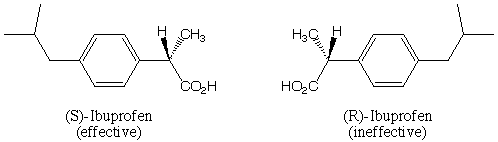
The drug thalidomide was introduced in Germany in 1957 as a sedative.
It was soon found that it relieved the symptoms of morning sickness in pregnant
women, and in the late 1950's and early 1960's was widely and successfully
prescribed in Europe for this use. At the time, the precedures governing
the application for approval of new drugs were much less rigorous than today.
The drug had undergone testing in rodents, but not in higher mammals. The
drug companies wanted to gain approval to use the drug in the US, the largest
market in the world. The application was handled by a junior medical reviewing
officer in the FDA, Frances Kelsey. Because the drug was already in widespread
use in Europe, and because this was the very first case for Kasey, a simple
approval was expected. However, Kelsey was not so easily convinced and after
careful review of the application she decided that further research was
required before she could approve the drug. For example, she noted that
although the drug resulted in no harmful effects in rodents, it did not
have the sedative effect either! This suggested that rodents were not good
test subjects for determine the effects in humans. She was under tremendous
pressure to grant approval, but stubbornly refused to yield. By holding
so firm to her position, she turned out to be a true hero.
In 1960, doctors
started to notice an extremely horrific side-effect related to the use of
the drug. Some of the pregnant women who took thalidomide had babies with
terrible defects, typically limbs were badly deformed or completely missing,
and the babies often also had mental illnesses. The drug was quickly withdrawn
in 1961. Over 12,000 "thalidomide babies" were born, but thanks
to Frances Kelsey only 17 of these were in the US. It is thought that those
in the US were a consequence of women obtaining the drug themselves from
overseas. Of these 12,000, only 8,000 initially survived. Although many
went on to lead fulfilling lives, their life expectancies were also reduced and there only two or three thousand alive today.
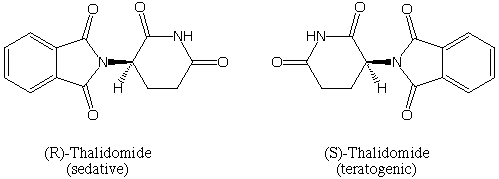
Subsequent testing in mammals revealed a remarkable result that it was the (R)-form that exhibited the sedative effects, but the (S)-form was a potent teratogen that caused the terrible fetal abnormalities. It turns out that even if this had been recognized at the time, and only the (R)-form of the drug had been administered, the results would have been the same. This is because like the ibuprofen example above, the (R)- and (S)- forms interconvert in the body.
Most of the thalidomide babies were born at the same time that I was. Growing up in Europe in the 1960's I saw several thalidomide babies, they were basically my peer group. This was a horrible tragedy, even now it is difficult for me to think about it. This event lead to the introduction of a much more rigorous drug approval process both in Europe and in the US. In particular, the "handedness" of any new drugs must be specifically tested. As we will see in class, it is often very difficult to synthesize a drug in one specific "hand" ((R)- or (S)-). This has lead to a large area of research in chemistry related to the development of methods to synthesize molecules with specific "handedness". This has also contributed to what some people think are excessive delays in granting approval for new drugs. This is obviously not a simple matter.
This isn't so much a story about enantiomerism as it is about good science and conviction. Frances Kelsey is a hero.
Remarkably, Thalidomide is still under investigation for possible therapeutic benefits. For example, it has been found to be extremely effective in the treatment of leprosy and other inflammatory diseases. It has also been used to treat weight loss associated with AIDS/HIV and on Chron's disease patients. I have read that women are not even allowed to enter one of the factories where thalidomide is now being manufactured.