|
Chirality |
Molecular "Handedness"
|
|
|
|
� Stereoisomers differ in the orientation of their atoms in space, we have seen cis/trans isomers (Diastereomers)
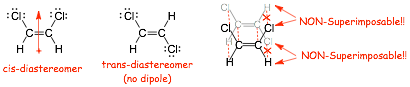
� DIASTEREOMERS have same molecular formula but different physical and chemical properties, they are different molecules. They are also NON-SUPERIMPOSABLE
|
1 Enantiomers |
� Important NEW type of stereoisomer is the ENANTIOMER
� Enantiomers are "mirror image" stereoisomers
� Enantiomers have the property of chirality
� Chiral objects have a "handedness"

� The molecule on the left is the enantiomer of that on the right, they are enantiomers
� Both isomers are chiral (they differ from their mirror image), in this case as a consequence of a "chiral center"
� A chiral center is an atom with four different groups attached to it (starred above)
� The presence of a chiral center (also known as an asymmetric center) is the most common reason for a molecule to be chiral
LOTS OF THINGS can be chiral, this is NOT only a property of molecules
� hands are chiral, screws are chiral, there are many chiral things!

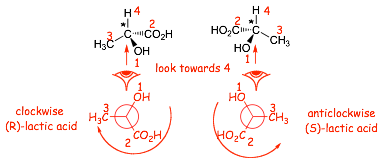
Example: lactic acid (the chiral/asymmetric centers are starred)

Example: 4-bromooctane

� switch any two substituents on a chiral center to get the enantiomer, see BELOW

Example: substituted cyclohexane
� Recall, we had to be very careful in placing the substituents on cyclohexanes properly

|
1.1 Specifying Configuration (Chirality); Cahn-Ingold-Prelog Notation |
� Specifies the "configuration" at each asymmetric or chiral/asymmetric center, equivalent to "cis-" and "trans-"
� Same rules used to decide priority of the substituent groups as used when determining Z and E in alkenes
1) Assign priorities to groups attached to chiral center according to atomic #
2) Compare 2nd, 3rd etc. atom from center as necessary, look for first point of difference
3). Multiple bonds "add up"
4) Determine direction of rotation 1 > 2 > 3 looking with #4 group pointing "away"
Example
Example
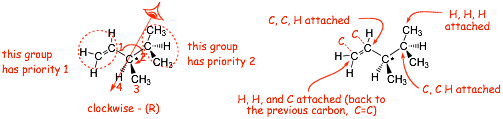
� treat each double bond as an additional single bond to count the 3 atoms that are further connected to each carbon, so in this case the -CH=CH2 "wins" over the CH(CH3)2
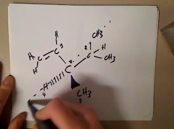
Visualize how to Determine the direction of rotation using your hand
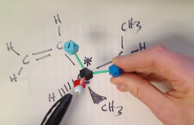
Visualize how to Determine the direction of rotation using a model
Example: name the following compounds

� note that 2,3-dibromohexane is the name for several different stereoisomers, both enantiomers and diastereomers, need to specify the absolute configuration, R or S, at each chiral center to correctly name THIS stereoisomer
� NOTE: R and S are ALWAYS capital letters!
NOTE: the lowest priority group does not have to be on a wedged or dashed bond

� the bond to group 4 is in the PLANE of the paper this time
� ALSO, once we have determined that the structure on the left (above) has the absolute configuration (R) we don't have to do the same for the left hand structure since it is the mirror image isomer, and the mirror image isomer MUST then be (S)
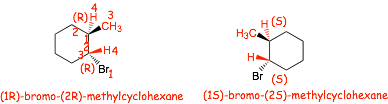
� note that trans-1-bromo-2-methylcyclohexane is an INSUFFICIENT NAME since it describes BOTH!!
|
1.2 Consequences of Chirality |
� The physical and chemical properties of diastereomers are DIFFERENT, e.g.

� HOWEVER, the physical properties of enantiomers, m.p. b.p. dipole moment etc. are the same
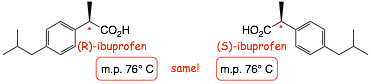
� Chemical reactions of enantiomers are ALSO the same, except for reactions with other chiral molecules. This is extremely important in biological systems, all but one of the amino acids are chiral, all enzymes are chiral and even DNA has "handedness", only one enantiomer of a chiral drug is generally active etc.
Example: ibuprofen is sold as a mixture of enantiomers, but only the (S) stereoisomer is actually active

Another famous example is the two enantiomers of the drug thalidomide, one enantiomer is a sedative but the other enantiomer is a teratogen (causes birth defects), thalidomide as a drug was inadvertently the cause of many terrible birth defects in the 1950's (actually it is a bit more complicated than this, but the details must be described elsewhere�.)

The optical activity of enantiomers, is different
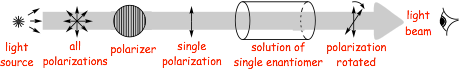
� optically active solutions rotate plane polarized light
� opposite enantiomers (R) (S) rotate light in equal and opposite directions (they have opposite optical activity)
� enantiomers of different compounds rotate light to different extents
� the extent of rotation depends upon the particular enantiomer, the concentration in solution and the optical path length
� solutions that rotate to the left called levorotatory, symbolized by ( �)
� solutions that rotate to the right called dextrorotatory, symbolized by ( +)
There is no relationship between (R) & (S) and (+) & (�)
For Example
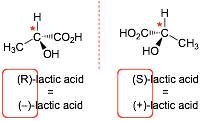
(S)-lactic acid rotates light to the right, and so is (+)-lactic acid
(R)-lactic acid rotates light to the left, and so is (-)-lactic acid
But
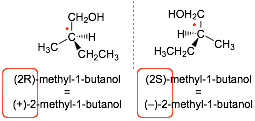
(2S)-methyl-1-butanol rotates light to the left, and so is (�)-2-methyl-1-butanol
(2R)-methyl-1-butanol rotates light to the right, and so is (+)-2-methyl-1-butanol
* There is no relationship between R/S and + and �
|
1.3 Racemic Mixtures |
� are exact 50 : 50 mixtures of opposite enantiomers, symbolized by (�)
� racemic mixtures are optically INACTIVE because the optical activity of one enantiomer is exactly cancelled by the optical activity in the other enantiomer

� Represent a racemic mixture as the TWO structures with wedged/dashed bonds, OR, as the single structure with NO wedged/dashed bonds and the (�) symbol
� It must seem that a racemic mixture, being exactly 50:50 would be unlikely, or difficult to prepare exactly, but we will see that in fact racemic mixtures are quite common in organic chemical systems
|
1.4 Achiral Compounds With Asymmetric Centers: Meso Compounds |

� This molecule HAS chiral centers BUT is superimposable on its mirror image
� If it is superimposable then it CAN'T BE CHIRAL and its mirror image is NOT A STEREOISOMER
� The structure is achiral and a solution of it will not be optically active, it is a MESO COMPOUND
� This is because the molecule has a mirror plane itself
� Molecules with mirror symmetry planes can not be chiral!
� The presence of a chiral/asymmetric center is therefore an insufficient reason ALONE for chirality
Examples

Example: Is the following structure chiral?
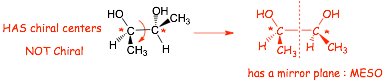
� the structure on the left above obviously does NOT have a mirror plane, however, if a structure with chiral centers has a mirror plane even in only ONE conformation, as shown on the right, then it will be a MESO COMPOUND. The way to think about this is that for each non-mirror plane conformation there will be another conformation that "cancels" the chirality
|
1.5 Multiple Chiral (Asymmetric) Centers |

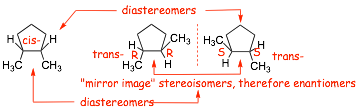
� Enantiomers have opposite configurations at ALL chiral centers!
� Diastereomers are stereoisomers that do NOT have opposite configurations at all chiral centers
Examples:

� Opposite configuration at ONE chiral center only (R/R versus S/R): these are diastereomers

� Opposite configuration BOTH chiral centers (R/R versus S/S): these are ENANTIOMERS
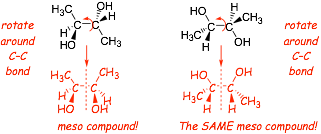
� In this case the structure on the right was ALSO rotated around the central C-C bond, identifying the kind of isomer visually is difficult in such cases, but assigning absolute configurations and comparing them makes it easy
Example :
what is the relationship between these two structures?
Summary
� A chiral compound is non-superimposable on its mirror image
� A chiral (asymmetric) center has four different groups attached to one atom
� An enantiomer is a "mirror image" stereoisomer
� A diastereomer is ANY stereoisomer that is not an enantiomer
� A meso compound has chiral centers but it not chiral (is achiral) due to a mirror plane of symmetry
|
3 Stereochemistry in Chemical Reactions |
� Previously we had to consider the stereochemistry of cis-/trans- isomers in organic reactions
� NOW, we ALSO have to consider ENANTIOMERS and CHIRALITY!
� HOW DO WE KNOW WHETHER WE ALSO need to consider chirality and enantiomers?
� If there are CHIRAL/ASYMMETRIC carbons/centers in they products then we need to consider these stereoisomers
Example 1
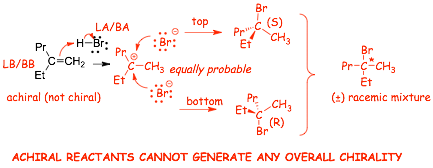
� This reaction does not make cis/trans isomers, but there is a CHIRAL CENTER in the product, therefore we need to consider CHIRAL ISOMERS
� A complete description of the products demands that we show that a pair of enantiomers is formed either by drawing both structures with wedged/dashed bonds, OR, a single structure with PLAIN bonds and (�)
� NOTE, do not include wedged/dashed bonds here if you use (�), they would be redundant, or even misleading
� achiral reactants must give achiral products, in this case a racemic mixture!
Example 2
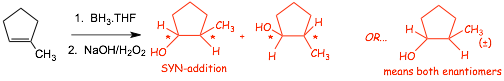
� There are CHIRAL CENTERS in the products, therefore we need to take into account cis-/trans isomers AND isomers related to CHIRALITY
� The products of this reaction CAN be cis/trans isomers, because the addition is SYN, the geometrical (cis/trans) isomer that has the two new -H and -OH bonds on the same side of the ring must be specified.
� ADDITIONALLY, two chiral centers are also formed and so ANOTHER form of stereoisomer must be considered, the enantiomer
� The starting alkene is achiral (it has TWO mirror planes, one vertical and one in the plane of the paper), and thus can not generate OVERALL chirality, thus a racemic mixture had to have been formed
� We also understand that a racemic mixture had to be formed because the -H and -OH could, and should add to both sides of the alkene with equal probability
� Conventionally, only ONE (either one) of the enantiomers is drawn, WITH the new -H and -OH bonds cis-with respect to each other, AND the (�) racemic mixture symbol is used. We need wedged/dashed bonds in addition to the (�) symbol in this case because there are TWO kinds of stereoisomers, the wedged/dashed bonds are needed here to specify that the addition was SYN, the (�) is need to specify that two enantiomers are formed
� TWO stereoisomeric products are formed, and a complete description of the products demands that we show both the relative stereochemistry (anti-addition using wedged/dashed bonds etc) AND that a pair of enantiomers is formed using (�)
Example 3
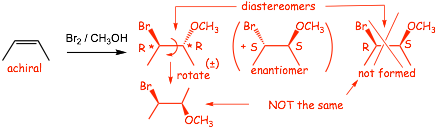
� There are obviously NO cis-/trans-isomers in this case, BUT there ARE CHIRAL CENTERS in the products, therefore we need to take into account isomers related to CHIRALITY
� BUT the starting alkene is achiral (it has TWO mirror planes, one vertical and one in the plane of the paper), and thus can not generate OVERALL chirality, thus there can be no overall chirality in the product(s)
� Either an ACHIRAL product, or a RACEMIC MIXTURE must be formed
� In this case the product is CHIRAL, therefore a RACEMIC MIXTURE must be formed
� We MUST include the wedged/dashed bonds in this case because we need to distinguish the (R,R) and (S,S) stereoisomers from the (R,S) and (S,R) diastereomers that are NOT FORMED
� Conventionally, only ONE (either one) of the enantiomers is drawn (in this example the enantiomer in the parenthesis could be omitted), the (�) racemic mixture symbol is used instead
� even though there is rotation around the central C-C bond, we must show the dashed/wedged bonds to differentiate the diastereomer that is formed from the one that isn�t formed (shown above)
� TWO stereoisomeric products are formed, and a complete description of the products demands that we show both the relative stereochemistry (anti-addition using wedged/dashed bonds etc) AND that a pair of enantiomers is formed using (�)
Example 4
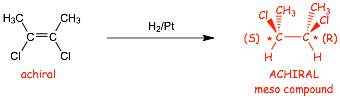
� There are obviously NO cis-/trans-isomers in this case, BUT there ARE CHIRAL CENTERS in the products, therefore we need to take into account isomers related to CHIRALITY
� BUT the starting alkene is achiral (it has TWO mirror planes, one vertical and one in the plane of the paper), and thus can not generate OVERALL chirality, thus there can be no overall chirality in the product(s)
� Either an ACHIRAL product, or a RACEMIC MIXTURE must be formed
� In this case the product is an ACHIRAL MESO COMPOUND
� We STILL MUST include the wedged/dashed bonds in this case because we need to distinguish the (S,R) meso-stereoisomers from the (S,S) and (R,R) stereoisomers that are NOT FORMED
� even though there is rotation around the central C-C bond, we must show the dashed/wedged bonds to differentiate the stereoisomer that is formed from those that do not form
Different Example

Q. is a solution of the product of this reaction optically active?
A. it depends upon the mechanism!
Mechanism (A) : 1 step, transition state (�) but no intermediate
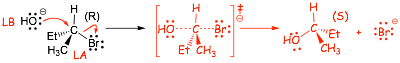
� pure enantiomer formed, product solution is optically active
� new bond made at same time as old bond broken : inversion of configuration
Mechanism (B) : 2 steps with intermediate
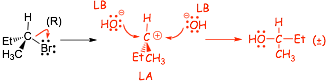
� attack from both sides equally probable
� racemic product mixture formed
� product solution optically inactive!
� it is possible to distinguish between these mechanisms by determining the optical activity (or lack of) in the product solution.