Organic Chemistry in "Real Life"
In this section of the notes we talk about bonding molecular orbitals as the place "where the electrons are" in molecules. What about those seemingly useless antibonding M.O.'s? They usually don't have any electrons in them so why do we care about them? For several reasons. First, we need to understand that the wavelike nature of the electrons results in wavefunctions that must have both positive and negative phase. Thus, there must always be two ways of combining atomic orbitals, in and out of phase. Thus, by knowing about anti-bonding orbitals you are necessarily understanding the wave-like nature of orbitals. Second, we will soon see that when molecules undergo reactions, electrons are usually "donated" from one reactant to the other, and the reactant that "recieves" the electrons must accept them into an empty orbital, which for most molecules is an anti-bonding obital. Thus we will need to understand the properties of the anti-bonding orbitals that are associated with a particular bond, e.g. where the nodes are, on which atom is the orbital concentrated. This will help us to predict organic reactions.
There is another area in which the antibonding orbitals play an important role, i.e. in determining the colors of molecules. Why are things colored? To understand this you need to know something about light.
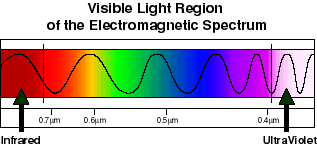
Light that we can see is an example of electromagnetic radiation, more specifically it represents that part of the electromagnetic spectrum that our eyes are sensitive to. The difference between red light and blue light (for example) is the frequency of the electromagnetic oscillations. Red light has low frequency or (relatively) long wavelength oscillations, blue has higher frequency or (relatively) short wavelength oscillations. Red represents the lowest frequency (longest wavelength) that we can see. Light that has lower frequency (longer wavelength) oscillations than red light is infrared. Blue represents the highest frequency (shortest wavelength) we can see. Light that has higher frequency (shorter wavelength) oscilaltions than blue light is ultraviolet. Green light is in the middle of the range of frequencies (wavelengths) that we can see. The wavelength of red light is roughly 0.7 μm, or 700 nanometers. Blue light is roughly 0.4 μm, or 400 nm. White light is a mixture of blue, green, red etc light. White light can be split into its constituent colors by passing it through a prism. The energy of light is directly proportional to its frequency. Low frequency (e.g. red) light has a (relatively) low energy. High frequency (e.g. blue) light has a (relatively) high energy.
|
|
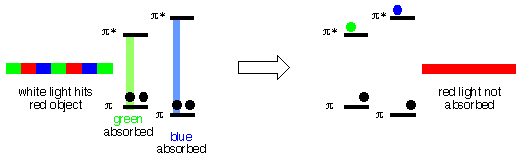
When white light (the mixture of colors) from a light bulb, the sun etc., shines on an object that is red, the red part of the white light is reflected off the object to your eyes. The blue and the green parts do not reflect, they are "absorbed" by the object. A blue object reflects only blue light (not red or green), a green object absorbs red and blue, etc. Other colors are "made" by mixing the primary colors in different proportions etc. A black object absorbs all wavelengths of light that hits it, a white object reflects all wavelengths of light that hits it.

So, how is the light absorbed? The light energy has to go somewhere, it has to be "used". It turns out that the energy of light in the visible region can be close to the energy difference between bonding and antibonding Molecular Orbitals in molecules. When light is absorbed, the energy is used to move an electron from a low energy bonding (usually π) M.O. to a higher energy antibonding (π*) M.O. If the energy difference between a bonding π M.O. and its corresponding π* M.O. is equal to the energy of (for example) green light, then the green light can be absorbed. If the energy difference between a bonding π M.O. and its corresponding π* M.O. is equal to the energy of (for example) blue light, then the blue light can be absorbed. In the example below, there is no bonding π M.O./π* M.O. pair for which the energy difference is the same as that of red light, so the red light is not absorbed.
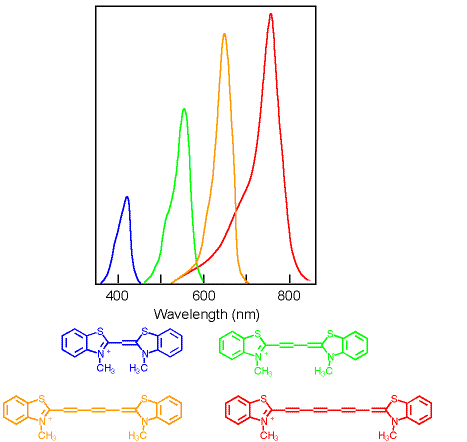
Chemists can control the energy difference between the π M.O. and π* M.O.'s to control the colors of the light absorbed. This is how dye chemistry works! In general, the energy difference gets smaller the bigger the molecule (we will talk a lot more about this next semester). Here are some dye molecules that are used in photographic film. The different dyes make the film sensitive to different colors of light, blue, green, orange, red etc. The figure below shows how much the different dyes absorb the different wavelengths of light.
What happens to the electrons once they are "promoted" into the antibonding M.O.'s. As you can imagine, they are very reactive, being so high in energy. The energy of these electrons can be used to initiate many useful reactions, such as those in imaging such as photography or xerography. If the light that hits the molecules is sunlight then they can also be used to initiate chemical reactions that can store the light energy in solar conversion schemes. If the light is sunlight and the dyes are in plants, then the light absorption can be used to initiate the photosynthesis process. So you see, the antibonding orbitals are used in the most important chemical processes, those responsible for life itsself :)
These reactions we have just talked about come under the umbrella of photochemical reactions, which is what I work on in my own research lab. In fact I used to earn my living as a photochemist at the Eastman Kodak Company. ASU is home to the Center for the Study of the Early Events in Photosynthesis. You might like to check out their web site.