
Organic Chemistry in "Real Life" : What is Organic Chemistry?
Every Organic Chemistry Textbook starts with this question. The standard answer is pretty concise and simple, it is that branch of chemistry that describes the structure, properties, and reactions of compounds that contain carbon. That says a lot, and at the same time sort of doesn't, and so the textbooks then go on to give a list of the sub-branch of organic chemistry then examples of important organic chemicals, polymers, pharmaceuticals, biomolecules etc.
I am not going to repeat all of this here, anyway, you can easily look up this standard stuff in the source of all knowledge for students, Wikipedia!
Instead, here is a very personal view of what organic chemistry is, in the form of a brief overview of some of the organic molecules that I have studied in my research career, and why.....
This is methylene, it is just about as small and simple as an organic molecule can be. It is very reactive because it has only two bonds to carbon. It is of fundamental interest to chemists because it is so small and so reactive. It had never been properly studied in solution because it is so reactive it will react with just about any solvent. We found a way to study methylene in a very unreactive solvent and were the first to determine the kinetics of its reactions in solution.

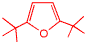
This is di-tertiarybutlyfuran, it is the first molecule that I had to synthesize as a graduate student that had not been previously been reported. We were interested in this molecule because we were trying to understand how singlet oxygen reacts with organic molecules. At the time, singlet oxygen was a big deal in the mechanistic organic chemistry community and exactly how its reactions occurred wasn't known. We worked hard on this problem but didn't really resolve it. I think that I know the answer to this mechanistic problem now, but not as many chemists are interested in this now!
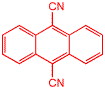
This is dicyanoanthracene, one of my favorite molecules. We worked a lot with dicyanoanthracene when we were studying how the simplest organic reaction of all occurs, i.e. single electron transfer from one molecule to another. Single electron transfer is one of the primary processes in complex organic reactions, and how it worked, or more exactly the connection between observations and theory, was very mysterious. We were able to contribute to this problem in a series of papers published over nearly ten years, including finding that some electron transfer reactions get slower the more exothermic they get! Almost every one of these papers involved dicyanoanthracene in one way or another!
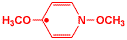
This is a radical, we will learn what a radical is later in the course. We were trying to find reactions that occurred at the fastest possible rate, so that the molecule would have no lifetime at all, i.e. it would react as soon as it was formed. There are very good practical applications for such chemistry if it can be made practical. This radical has a lifetime that is so short that it could not be measured on the ASU femtosecond laser apparatus, which means that it lives less than a thousandth of a billionth of a second. We also studied it theoretically inside a computer and found that indeed it has no lifetime at all.
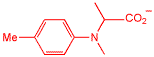
This is an unnatural amino acid, it doesn't play any role in biology, but it was designed to respond to light in such a way that it liberates two electrons when activated. We designed this structure to work in a photographic emulsion, basically, it makes photographic film twice as sensitive to light than normal. I worked on this chemistry when I was at the Eastman Kodak Company.
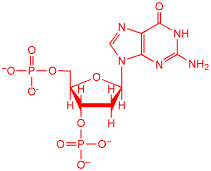
This is the guanine nucleotide, that is one of the four bases in DNA. When DNA is oxidatively damaged, the primary intermediate is an oxidized form of guanine, actually, the guanine radical cation. The reactions of guanine radical cation in DNA are difficult to study. We found a photochemical method to form guanine radical cation in DNA and was able to observe it's primary very fast reactions for the first time.
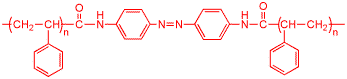
This is a modified form of polystyrene. Polymers have repeating units of organic molecules that can contain millions of atoms, they are very large "molecules". The structure shown below is the repeating unit. The polystyrene has an azobenzene structure inserted (the part with the two benzene rings that surround the two nitrogens joined by the double bond). The azobenzene "probes" the extent of free volume in a polymer film. Free volume is one of the important theoretical concepts in polymer chemistry, and this molecule introduced a new way of measuring this.
So, there you go, a small piece of what organic chemistry is to me at least, from the very small (molecule) to very large. What you may have gathered from the brief descriptions above is that most of the time that I have spent in organic research has been directed towards understanding how things work, and this has influenced the way that I teach.
For you, I hope that this emphasis on understanding will mean that organic chemistry is a subject that makes sense, that it CAN be understood, and that via understanding, your learning will be more enduring (after all, the MCAT and equivalent exams are on the horizon for many of you!) and perhaps even enjoyable.