O-Chem in Real Life: Amines and Photography
I once spent a lot of time working on the chemistry of amines! At Eastman Kodak we worked on the development of a new method for photography. The chemistry is a bit complicated, and is well-beyond normal General Organic Chemistry course material, but I'll try to explain it anyway.
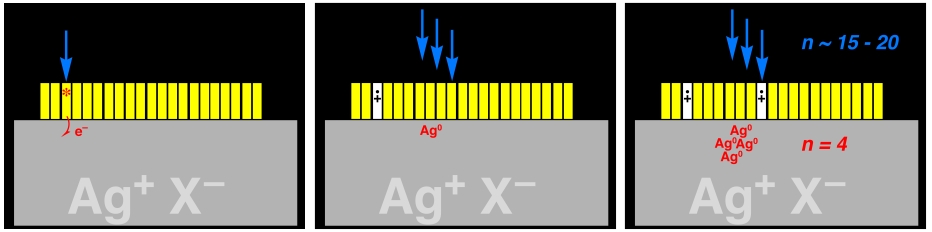
We need to start by understanding some of the basics of how normal photography works. Photographic film consists of millions of microscopic crystals of crystalline silver chloride and/or bromide (Ag+X-) that are "coated" with a layer of a colored dye that is one molecule thick. When the film is exposed to light, the dye molecule absorbs the light, forming a high energy form of the dye which is called an excited state and is indicated by *. The dye exited state then "gives" an electron (e-) to the silver halide crystal.
Exposure to light will result in many of these excited state dyes being formed, and many electrons being delivered to the silver halide. The film only needs to be exposed to enough light for 4 electrons to be delivered overall to the crystal. Any crystal that has received enough light to get 4 electrons is considered to be properly exposed, and will develop into part of the colored image when the film is developed. Crystals that receive less than 4 electrons will not develop, and will contribute to dark areas of the final image.
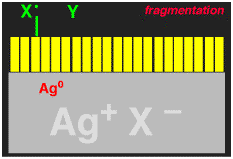
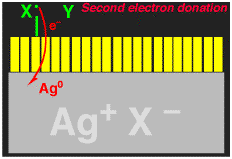
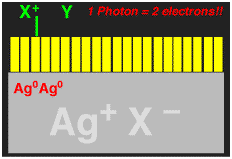
This exposure process is illustrated schematically in the following pictures, where the large grey object is a blown-up picture of a silver microhalide crystal, the yellow rectangles represent individual dye molecules that are absorbed on the surface. The arrows represent the light hitting the crystal, and the Ag0 represent the electrons that are captured by the crystal. Capture of an electron turns one silver cation into neutral (i.e. metallic) silver, Ag0.

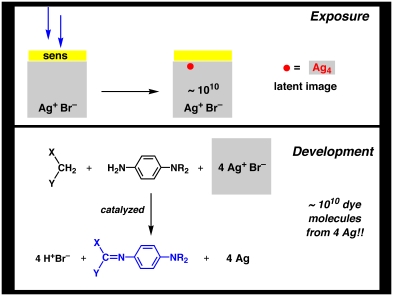
What happens in the development process is almost magic! In the developer solution, those crystals that have been properly exposed (the technical term is "contain a latent image"), catalyze formation of the colored image dye molecules that you see in the final image. It takes 4 silver ions to make one dye molecule. However, for the exposed crystals, ALL of the silver ions in the crystal can get involved in making dye molecules. Because there are billions of silver ions in each microcrystal, billions of image dye molecules can be formed from only the 4 electrons that were initially captured. It would be impossible to "see" the 4 silver atoms, but it is obviously possible to see the billions of image dye molecules that are formed upon development of each crystal. This process is illustrated schematically below.
The development process thus represents a huge chemical amplification step. Incidentally, it is this chemical amplification property that ensures that the electronic sensors in digital cameras will never be as efficient as photographic film.
So, where do the amines come into this? Even though photographic film is very sensitive, there was always a need for even more sensitive film. A more sensitive film would work with shorter exposure times, or would work in lower lighting situations, or would work with small lenses, etc. The question is, how to make film even more sensitive?
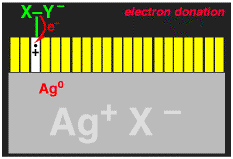
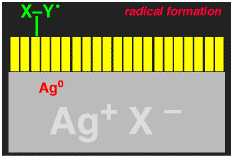
We wanted to make the sensitization (electron injection into the silver halide crystal) process more efficient. When an electron is transferred from the dye to the silver halide, what is left behind is a dye minus an electron, i.e. a cation (actually it is a radical cation). The dye radical cation has chemical potential energy that was being wasted, we wanted to use it! We added another molecule, let's call it generically X-Y for now, that would give an electron to the dye radical cation. If the X-Y molecule is negatively charged, i.e. an anion X-Y-, then after losing one electron to the dye it becomes a neutral radical, X-Y.. The X-Y. had to be designed to that it would spontaneously break apart to give an X. radical, with liberation of a small neutral molecule Y. Finally, the X. radical had to be designed so that it would want to give an electron to the silver halide, to generate an Y+ cation.
The entire reaction sequence for the X-Y- is shown below. If this could be made to work, then TWO electrons could be delivered into the silver halide for each light photon absorbed, thus DOUBLING the photographic sensitivity of the film. We called the new process "Two-Electron Sensitization".
The final issue is the actual structure of the X-Y- molecule. Giving one electron is like giving two, so a molecule was required that had the properties of a good nucleophile or good Lewis base. We know that for neutral molecules, amines are good nucleophiles, so this was our start.
The photographic medium is mainly water at around pH = 7. The amine could not be protonated, so we needed an amine with pKa less than 7 (remember, for amines this value refers to that of the protonated form!), which meant we required an aromatic amine. The amine also had to dissolve in water, and so negatively-charged carboxylate groups were built into the structure to ensure water solubility. The amine had to fragment after it had donated an electron to the dye. We have learned that reactions that form small simple neutral molecules tend to be efficient, and so we designed the aromatic amine so that it would fragment to give carbon dioxide (CO2). Finally, the amine radical had to give an electron to the silver halide. When an amine radical gives an electron it forms an iminium salt (we have seen these in second semester organic). The more stable the iminium salt the more likely the radical is to give the electron, so we designed amines with electron donating groups to stabilize the positively charged iminium salt. The entire Two-Electron Sensitization process for an amine that is useful in a photographic system is shown below.
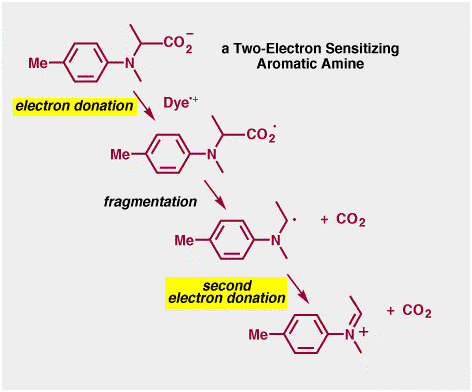
So, does it work? Well, actually it does!! Compounds such as those above really do enhance photographic sensitivity when added to film. We actually made probably around a hundred or so anologues to the molecule above, since we actually needed pretty fine control over the electron donating abilities, the fragmentation rate, the solubility etc. The Two-Electron Sensitization process is actually a bit too sensitive for use in consumer film, but it is used in motion picture film.
The Two-Electron Sensitization Concept is protected by US Patent Numbers 5,747,235, 5,747,236, 5,994,051, 6,010,841, 6,054,260, 6,153,371 and 6,306,570. It is also probably one of the last innovations in photographic chemistry. The reason for this is pretty easy to understand. Nobody buys film anymore and research in this area is essentially completely ceased, which one of the reasons that I am now teaching at ASU!