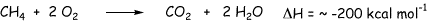
Organic Chemistry in "Real Life" : Burning Hydrocarbons
Simple hydrocarbon organic molecules, alkanes, are chains and rings of carbon atoms saturated with hydrogens. Alkanes represent the "backbone" of organic molecules, the scaffold upon which the functional groups that do the interesting chemistry are built. Alkanes get involved in almost no interesting chemistry, but are very important in determining the shapes and sizes of organic molecules. One of the few chemical process involving hydrocarbons is combustion!
The small hydrocarbons, methane ethane propane butane, are gaseous fuels and in fact methane in the primary constituent of natural gas (ca. 75%). Larger hydrocarbons containing roughly 7 to 10 carbons are the primary constituents of gasoline. Even larger hydrocarbons (greater than 12 carbons) are used in diesel fuels. Hydrocarbons can burn completely to give carbon dioxide and water. This reaction is very exothermic. Burning 1 mole of methane generates about 200 kcal per mole of heat, that is a lot!
Where does this heat come from? You know the answer, it is the usual one, from the energy of the electrons! We will be talking about how and why chemical reactions generate heat in more detail soon in class. One of the important contributing factors is the energies of the bonds. The sum of the bond dissociation energies for carbon dioxide and two water molecules (the products) is greater than that for methane and and two oxygen molecules (the reactants). The overall energy of the electrons in the bonds in methane and oxygen is higher than in carbon dioxide and water. Thus conversion of methane and two oxygens into carbon dioxide and two waters is quite exothermic and the reaction gives off (a lot of) heat, that can be used to (for example) boil water to turn a turbine in a generating plant.
What is heat? It is the kinetic energy of molecules (how fast they move). The molecules in a hot gas move faster and thus have a higher pressure in a fixed volume container. So, burning methane in the cylinder of an internal combustion engine moves the pistons due to the increased pressure of the hot gas products. Longer chain hydrocarbons represent more convenient fuels because they are liquids (they are more dense, more fuel molecules per unit volume). They also provide the opportunity to "control" the burning process. The chemical reactions involved in burning hydrocarbons are horrendously complicated, and I could find no simple description. However, radical reactions of the kind shown below are clearly important. Carbons centered radicals from the hydrocarbons, R., react with oxygen to give peroxy radicals, ROO., that give peroxides (ROOH). The oxygen-oxygen bond is particularly weak, and breaks easily at high temperatures to form more radicals. RO. . Radical chain processes result (we will talk about these later in the semester).
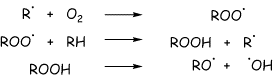
These reactions can be very fast and are very exothermic. They "feed on themselves" and under the correct conditions an explosion can result. This is NOT what you want to occur in your car engine! Such an explosion in your cylinder leads to "knock", rather than the controlled burn required for smooth operation. Different hydrocarbons have different tendencies to explode versus burn smoothly. For example, the straight chain heptane "knocks" very badly in car engines, but the branched 2,2,4-trimethylpentane (isooctane) burns smoothly. I really don't know why this is, but based on the chemistry above I can make a guess. An explosion results when the radical chain reactions occur very rapidly. Anti-knock ingredients in gasoline (for example lead compounds) are radical traps that terminate the chain reactions ( we will talk about this later too), to slow the overall process. So, I presume that the radical reactions involving isooctane are slower than for heptane, and do not require chain terminating agents to keep them under control. This is presumably because the radicals in the more branched isooctane are more substituted and hence more stable (another thing we will talk about later). They are also somewhat more sterically hindered (again to be discussed later). Overall these effects should lower the rates of the radical reactions in this case, which presumably results in more controlled (relatively slower) burning.
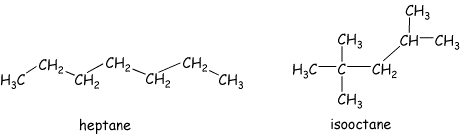
Gasolines are extremely complex mixtures of hydrocarbons. The compositions are varied to control many characteristics, one of which is knock. Higher octane fuels do not simply have higher amounts of octane in the mixtures! The octane rating is determined using an arbitrary scale (surely no chemist was involved in this!). Heptane, which knocks badly, is given an octane rating of zero, and isooctane, which burns smoothly, is given a rating of 100. Normal gasolines have octane ratings of around 86. This means that their complex mixtures burn the same way that a mixture of 86% isooctane and 14% heptane would. Gasolines often incorporate oxygen containing molecules, such as the infamous MTBE or ethanol, to aid complete combustion to carbond dioxide and water. Incomplete combustion leads to small carbon containing compounds that contribute to soot.
Gasoline is obtained by distillation of crude oil. The C7 - C10 hydrocarbons distillates have too many straight chain components, and a reforming process is applied to the distillates, that involves high temperature and catalysts to remove hydrogen from some molecules, dehydrogenate others, and break the carbon-carbon bonds in the long chains to make shorter and branched chains. The more branched structures can be used in lead-free gasoline. Gasoline chemistry is very complex!