|
Alkanes |
Introduction to 3D Structures
|
|
|
|
� Alkanes are hydrocarbons, i.e. organic molecules that contain only carbon (C) and hydrogen (H) atoms
� Alkanes are unsaturated (have no double/triple bonds), but may have rings
� Alkanes are generally unreactive and form the "backbone" skeleton of most organic molecules
|
1 Basic Organic Nomenclature |
Two kinds:
� Common or trivial names
� IUPAC (International Union of Pure and Applied Chemists!), systematic naming system
|
1.1 Basic IUPAC Rules |
1) find the longest (main) chain with the maximum number of substituents
2) number to give first substituent lowest number (then next lowest etc): look for first point of difference, and ONLY IF all other things are equal, number them alphabetically (but ONLY IF ALL ELSE is equal)
3) name substituents as "alkyl"
4) multiple substituents use the di-, tri-, tetra- etc. prefixes
5) substituents ordered alphabetically in the final name
� ignore di-, tri-, (unless part of complex substituent!) and also sec- and tert- and other prefixes that are hyphenated when part of a name
� do NOT ignore iso- and cyclo- (because these are NOT hyphenated when part of a name)
6) Complex substituents are numbered so that the #1 carbon as the one that is attached to the main chain
Example
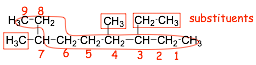
1) longest chain = 9, therefore a substituted nonane
2) start numbering from right, gives lowest numbers for substituents
3) 2 methyl substituents, one ethyl substituent
4) dimethyl and ethyl substituents
![]()
|
1.2 Nomenclature Terms You Are REQUIRED to Know |
|
methane |
|
|
methyl |
-Me |
|
|
|
|
ethane |
|
|
ethyl |
-Et |
|
|
|
|
propane |
|
|
propyl |
-Pr |
|
cyclopropyl |
|
|
|
|
|
isopropyl |
-i-Pr |
|
|
|
|
butane |
C4H10 |
|
butyl |
-Bu |
|
cyclobutyl |
|
|
|
|
|
sec-butyl |
-sec-Bu |
|
|
|
|
|
|
|
isobutyl |
-i-Bu |
|
|
|
|
|
|
|
tert-butyl |
-t-Bu |
|
|
|
|
pentane |
|
|
|
|
|
cyclopentyl |
|
|
hexane |
|
|
|
|
|
cyclohexyl |
|
|
heptane |
|
|
|
|
|
as above |
|
|
octane |
|
|
|
|
|
as above |
|
|
nonane |
|
|
|
|
|
as above |
|
|
decane |
|
|
|
|
|
as above |
|
|
undecane |
|
|
|
|
|
as above |
|
|
dodecane |
|
|
|
|
|
as above |
|
Some other substituents that you are also required to know��..
![]()
� the halogens are straightforward
� when the aromatic benzene ring is a substituent it is a phenyl substituent (-Ph)
Example
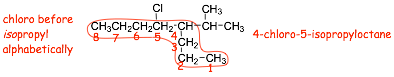
� HERE, the substituents get the same numbers numbering from EITHER END, 4 and 5. In this case then the substituents are numbered alphabetically, and because chloro comes before isopropyl alphabetically then we have 4-chloro and 5-isopropy.
� We ONLY number substituents alphabetically when ALL ELSE IS EQUAL, which is rare!
Example

� longest chain = 10, therefore a decane
� methyl in the #3-position
� complex substituent in the #6-position, this substituent is named according to IUPAC rules
� complex substituents are ALWAYS numbered so that the #1 carbon is the one that is attached to the main chain (the numbering isn't to give the substituents the lowest number in this case), number this way EVEN if this does not generate the longest possible chain
� the "di" in the complex substituent is not ignored alphabetically (di is ignored alphabetically in all other cases!)
Example
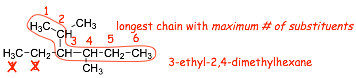
� When there are TWO possible longest chains, as in this case, choose the one that is MOST BRANCHED, i.e., has the largest number of substituents
Example

� look for the first point of difference, in this case the number of the second substituent
� therefore number starting at the left hand end (2,3,8,9,11) , even though numbering form the right (2,4,5,10,11) would "add up" to a smaller number
1.3 Cyclic Alkanes |
� The rules are the SAME, find the longest chain, if it is in a ring, then the main chain becomes a CYCLOalkane
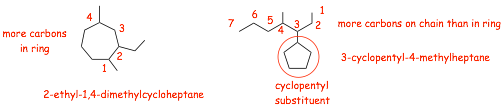
� if the longest chain is NOT in the ring, then the ring is the substituent (example on the right, above)
� We need to decide whether the longest chain is not in a ring, or in a ring, we don't look for a longest chain that includes both atoms in a chain and also outside a chain, the numbering wouldn't work well if we tried to do that!
1.4 Stereoisomers of Cyclic Alkanes |
� remember, a wedged bond means pointing "up" and a dashed bond means pointing "down"
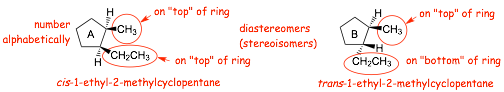
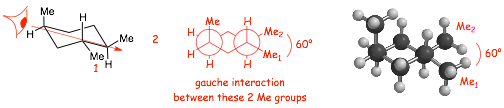
� in A, the methyl and ethyl groups both point "up", both are both on the same "side" of the cyclopentane ring (both on "top"), this is a cis-isomer
� in B, the methyl group points "up" and the ethyl group points "down", they are on opposite sides of the cyclopentane ring (one on "top" the other on the "bottom", this is a trans-isomer
� they differ in the way that the atoms point in space, they are a pair of STEREOISOMERS (diastereomers)
� NOTE, in the structure on the left the substituents were numbered ALPHABETICALLY (ethyl before methyl) ONLY because in this case EVERYTHING ELSE WAS EQUAL, the substituents would have been 1 and 2 whichever way we numbered
ALTERNATE Method for Drawing Ring Structures to Illustrate Stereochemistry
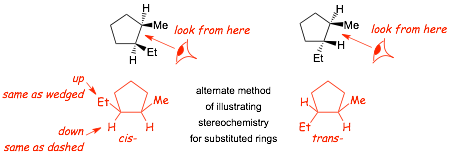
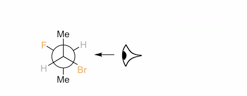
� "looking" from the side indicated by the eyeball clearly indicates which substituents are on "top" of the ring and which are on the "bottom"
� this alternate drawing method of representing stereochemistry on rings uses bonds that point up or down, to represent dashed/wedged bonds, assuming that you are looking at the structure towards the relevant bond
|
2 Conformations of Alkanes |
� A conformation is the arrangement of the atoms of a structure in space that can move as a result of rotation around a single covalent bond
� Conformations represents the different shapes that a structure has as a result of rotations around single bonds
|
2.1 Acyclic Alkanes |
Methane: Tetrahedral by VSEPR (remember: VSEPR minimizes the total energy of the electrons)
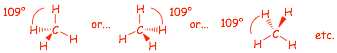
� Drawing the tetrahedral geometry in different orientations, but all have TWO plain and one wedged and one dashed bond
Ethane - Is conformationally "mobile", meaning that rotation around the single C-C bond is energetically facile and rapid. Below are shown two "3D" structures, using wedged/dashed bond formalism (also sometimes known as "sawhorse" structures) that illustrate the molecular geometry
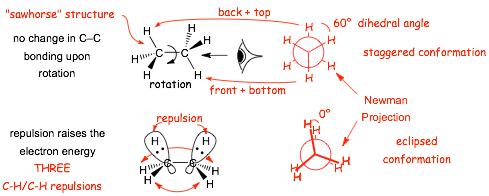
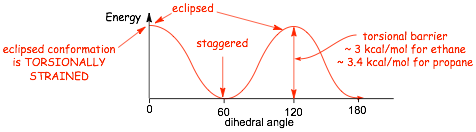
� the eclipsed conformation is higher in energy due to TORSIONAL STRAIN
Propane versus Ethane
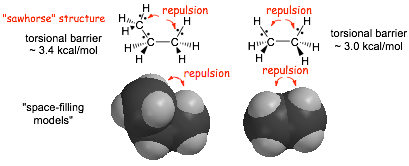
� �H vs. -CH3. results in more repulsion in the eclipsed conformation in propane compared to ethane
� propane has slightly more torsional STRAIN energy than ethane
Butane

� TWO unfavorable interactions in the fully eclipsed conformation of butane that contribute to torsional strain,
1) an electron repulsion effect, and 2) a steric effect
� A steric effect is the increase in electron energy that occurs when two sets of electrons attempt to occupy the same region of space
Visualize the Difference Between Electron Repulsion and the Steric Effect
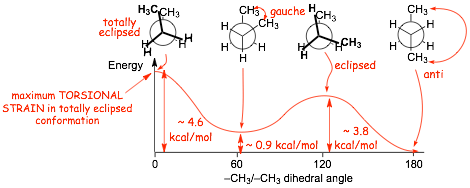
� although we identify and give special names to some of the conformations at energy minima and maxima, don't forget that there are actually an INFINITE number of conformations, and that at room temperature, butane will explore all of them pretty rapidly, although it WILL spend more time in the conformations at the energy minima,
Alkane Chains
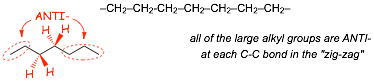
� When we draw alkyl chains in the usual "zig-zag" way, we are doing more than just indicating the positions of the carbon atoms, we are drawing the chain in such as way that ALL of the large alkyl groups are ANTI-with respect to each other for all of the C-C bond sin the chain, i.e. we are drawing the lowest energy conformation, the ALL-ANTI conformation
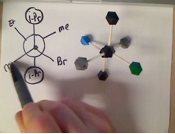
Example Problem: draw a 3-D sawhorse structure AND Newman projection for the lowest energy conformation for internal rotation around the C2-C3 bond in 3-methylpentane, looking from C2 to C3
� the Newman projection "looks" down the relevant bond
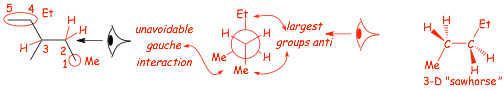
� the 3-D sawhorse structure "looks" at the relevant bond from the side

� either of the first two structures are good, they just have a slightly different perspective, the left hand one is tilted a little to the "right", the one in the middle is tilted a little to the "left", so that the atoms behind can be seen, the structure to the far right does not have reasonable perspective, the wedged/dashed bonds do not make sense
Example Problem: draw a Newman projection and 3-D sawhorse structure for the lowest energy conformation for internal rotation around the C3-C4 bond in 2,3,4,4-tetramethylhexane
� FIRST convert the IUPAC name into a line-angle structure�..
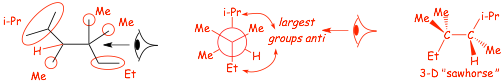
Visualize Drawing a 3-D/Sawhorse Structure from a Newman Projection
(and how to get the perspective correct)
2.2 Conformational Energy Barriers |
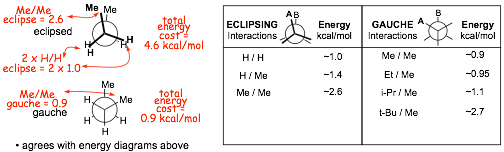
� the energy increases associated with electron repulsion and steric effects in alkane conformations can be quantified (see table). You should NOT memorize these numbers
Example Problem: Give the energy required to attain the fully eclipsed and gauche conformations of butane
|
2.3 Cyclic Alkanes |
Example: cyclopropane

� cyclopropane has considerable strain energy, due to:
1) eclipsing interactions (see Newman projection)
2) weak bonds (high energy electrons) due to poor overlap of A.O.s
Measuring strain energy using combustion analysis: Alkanes (e.g. propane) burn according to the following:
![]()
� The heat energy comes from the energy of the electrons in the C-H and C-C bonds of a hydrocarbon and the oxygen, for example, propane:

� the C=O and O-H bonds in the carbon dioxide and water are stronger that the C-H, C-C and O-O bonds in the propane and oxygen, stronger bonds means lower energy electrons, the electrons "go" from higher energy to lower energy upon burning, this electron energy is released in the form of heat (and light)
� the amount of heat that is released upon burning a hydrocarbon depends upon the number of C-C and C-H bonds, and so the generic formula for burning straight chain hydrocarbons is:

� Thus 3 x 157 = 471 kcal/mol would be expected be released for burning cyclopropane on this basis

� MORE energy is releas3ed than expected due to the STRAIN ENERGY in the cyclopropane
� the more strain energy molecule "has", the more heat is released upon burning
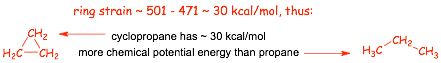
Strain Energies as a Function of Ring Size for Cycloalkanes
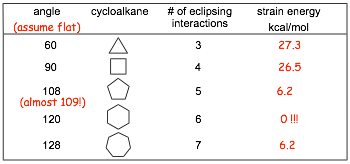
How do we explain the trends in strain energy in the table? Quite simply, the rings are not flat!!
Cyclobutane
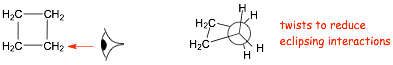
� ring "puckers" (twists) to decrease eclipsing, however, there are still 4 eclipsing interactions
� overall ring strain for cyclobutane similar to cyclopropane
Cyclopentane - adopts "envelope" structure
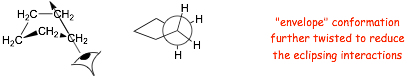
� The 5 eclipsing interactions are now small as a result of twisting into an "envelope" conformation, which is almost a staggered conformation (but not quite!)
� the envelope "migrates" around the ring, i.e. the carbon that "points up" rapidly changes position around the ring
|
2.4 Cyclohexanes |
� Cyclohexane has zero ring strain (minimal total electron energy!)
� the lowest energy conformation is the "chair"

� as the chair is drawn, 2 carbons are BEHIND the plane of the paper, 2 are IN the plane of the paper, and 2 are IN FRONT of the plane of the paper
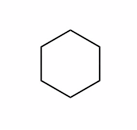
Visualize the Cyclohexane Chair
� the chair is COMPLETELY STAGGERED (it has zero eclipsing interactions)

� the chair has all bond angles ~109�, AND, it is completely staggered, which accounts for the zero ring strain, in turn this accounts for the very high frequency cyclohexanes and related 6-membered rings are encountered in nature
� another conformation is the boat
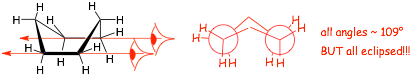
� the boat does have all bonds angles ~109�, BUT, it has almost all eclipsing interactions, and so it is a HIGH ENERGY conformation
Energetics of Interconversion of Chair Conformations
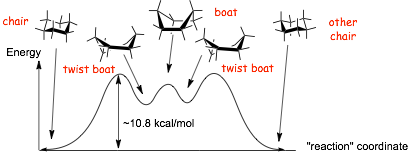
� Barrier to interconverting one chair to another ~ 10.8 kcal/mol
� Takes ca. 65 microseconds at room temperature
� even though this is still fast, it is a LOT slower than rotation around a single C-C bond in, for example, CH3�CH3
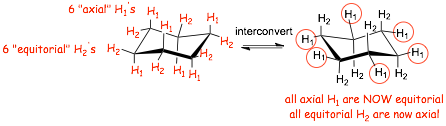
� interconversion between chairs converts ALL axial H's to equitorial and vica versa
Visualize the Chair-to-Chair Interconversion Using the HGS Model Kit
Drawing Chair Conformations
� a properly drawn chair consists of three parallel lines, don't draw a chair as shown below
� the AXIAL BONDS should all be straight UP on carbons that "point up", and straight DOWN on "down" carbons
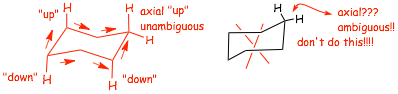
� the equitorials on the "left" point left, on the "right" point right
� the EQUITORIAL BONDS go "slightly down" on carbons where the axials are "up", and "slightly up" on carbons where the axials are "down"
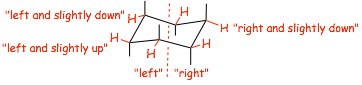
|
2.5 Monosubstituted Cyclohexanes |
� It costs ENERGY to have a substituent in the axial position compared to the equitorial position
Example: Ethylcyclohexane
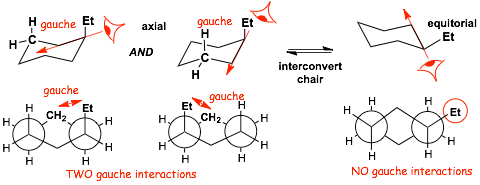
� TWO gauche interactions in the AXIAL conformation, zero gauche interactions in the equatorial conformation, THUS the AXIAL is HIGHER in ENERGY
Another look at this�
� TWO 1,3-diaxial interactions: which is another way of saying TWO gauche interactions
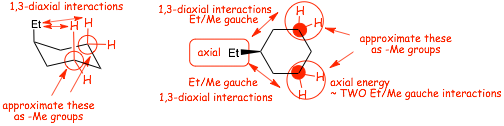
� gauche interactions in cyclohexanes are also referred to as "1,3-DIAXIAL interactions", since the arise as a result of electron REPULSION between the electrons in the bonds of the AXIAL substituent (ethyl here) and those in the AXIAL C-H bonds on the "3rd carbons" from the substituent
� the energy is roughly TWO Ethyl/Methyl gauche interactions
� We APPROXIMATE the #3 cyclohexane ring carbons as methyl groups
� the energy of a substituent in the AXIAL position is roughly 2 x a Substituent/-Me gauche interaction
Approximate the axial interaction to an R/Me gauche interaction, then can calculate the energy "cost"

Axial/Equitorial Equilibria for Monosubstituted Cyclohexanes

![]()
� t-butylcyclohexane essentially "locked" into the equitorial conformation
|
2.6 Disubstituted and Polysubstituted Cyclohexanes |
cis- and trans-isomers
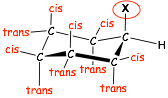
� with respect to the substituent X, which is "up", axial and equatorial substituents that are also "up" and cis-, axials and equitorials that are "down" are trans-
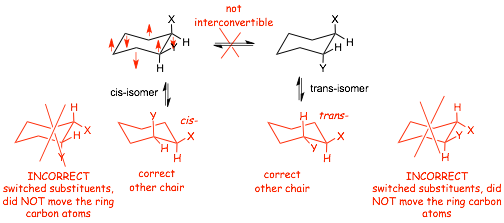
� cis- and trans- isomers are NOT interconvertible, however, EACH has two chair conformations
� when drawing other chair, it is VERY IMPORTANT to BOTH make all axials equatorial and vica versa, AND to interchange the positions of the carbon atoms. If you ONLY interchange the axials and equitorials without also changing the carbons, you will usually make a STEREOISOMER, i.e. a different structure
Visualize Drawing Correct Chair Conformations for Substituted Cyclohexanes
Example Problem: Identify lowest energy conformation of cis-1-chloro-4-t-butylcyclohexane

� Note that the assignment of the numbers to the carbons in the ring in the line-angle structure is arbitrary, #1 doesn't have to be at the top, but it is often convenient to put #1 at either the "top" or "bottom" positions
� Note ALSO that having carbon #1 on the "right" in the chairs is also arbitrary, but it is often convenient to do so (but not required!)
Example Problem: Identify the lowest energy conformation of the trans-1-isopropyl-3-methylcyclohexane structure provided�.
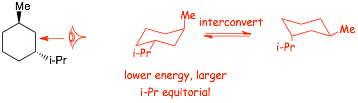
IMPORTANT, do not make the mistake of drawing the wrong enantiomer

� Changing the carbons that the substituents are attached to, especially "front" to "back" will often generate make a STEREOISOMER, i.e. a different structure
Visualize Chair-to-Chair Interconversions and Avoiding Common Misconceptions
Example Problem: Determine the energy difference between the lowest and highest energy conformations of the following structure (quantitative, not just qualitative)
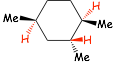
� we will need the table of gauche interactions that we used earlier

� remember, the energy associated with 1 axial interaction is equal to 2 gauche interactions
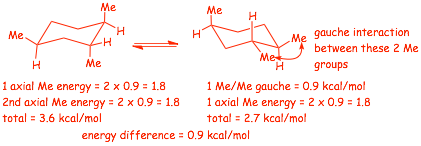
� Here we see ANOTHER form of "hidden" gauche interaction between the two -Me substituents when they are adjacent and both equatorial (also fairly easy to see in a molecular model)