O-Chem in Real Life: Hydrogen-Bonds
Alcohols represent the archetype polar protic solvents. The most characteristic feature of these solvents is their ability to donate and accept hydrogen bonds. Hydrogen bonds are obviously weak, they can readily be broken and reformed, and they play a central role in determining the properties of a particular kind of toy!
Silly Putty represents a nice example of the action of hydrogen-bonds, and also an example of scientific serendipity! During World War II, James Wright working for GE was trying to make a synthetic rubber. GE was a huge producer of silicone polymers, and so naturally Wright wanted to incorporate silicone into his new material. He was able to make material that was too soft to be used as a rubber, but because it was cheap and had interesting properties was not discarded. The new material was considered for many possible applications, but nobody really came up with a good one until it came to the attention of a Connecticut Store owner, Peter Hodgson. He bought a large amount, put it into plastic eggs, and sold it to children as a toy under the name Silly Putty!
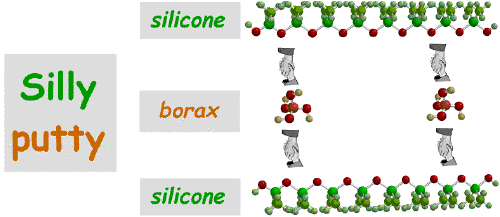
Silly Putty was originally made by combining the polymer silicone with borax, shown below. The figure shows the small borax molecules bonding to the silicone polymer, by "holding hands". This "holding hands" symbol is used because this is how we used to teach simple polymer chemistry to elementary school kids in an outreach program we used to run. The "holding hands" are really Hydrogen-Bonds!

It is the relatively weak hydrogen-bonds that contribute Silly Putty's properties. They can easily be broken and remade, allowing the silicone polymers to "slide" over each other.
You may have made "slime" or "silly putty" at home, school etc. It is a pretty common educational activity. The silly putty you can make at home is not the same as the commercial one, since it does not include the silicone polymer. Instead, the polyvinylalcohol that is the main component of white glue is usually used. This can be made into a silly putty by cross-linking with starch, which is itself a polymer (a polysaccharide), bristling with -OH bonds, or a slime can be made by cross-linking with borax. These are illustrated below, in the same "cartoon" way that the commercial Silly Putty was illustrated, for elementary school kids. When we make these polymers in our outreach lessons we call them ASU Putty and Irving Slime (when we used to go to Irving Elementary school in Mesa), to distinguish them from the commercial stuff.

These pictures are deceptive. Both of the "home made" materials contain a lot of water molecules that are really important in determining their properties (not shown). The polyvinyl alcohol almost certainly contains some polyvinylacetate, and ester, and covalent bonds may form in the crosslinks, but you get the idea!
Here are some pictures from our old outreach program



