O-Chem in Real Life: A Wittig Reaction
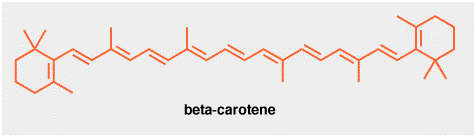
This is beta-carotene. It is a red colored natural product found in carrots, mangoes, sweet potatoes etc. Crystals of beta-carotene are so dark-red that they appear almost black. Good crystals are also shiny! How do I know this? I once spent quite a lot of time crystallizing beta-carotene, since it is used can be used to trap and quantify a photochemically reactive form of oxygen known as singlet oxygen, although we won't go into that here.

Beta-carotene is manufactured industrially using a Wittig reaction, i.e a reaction between a carbon-oxygen double bond and a phosphorous ylid, shown below. The aldehyde in this case is another natural product, vitamin A.
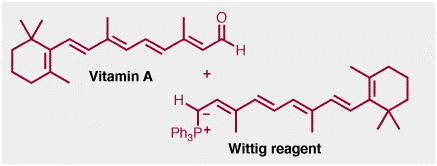
The Witting reaction is obviously not used in the biosynthesis of beta-carotene, in fact, the reverse ocurs. Vitamin A is formed by oxidation OF beta-carotene in the liver. Beta-carotene itself is an example of a tetraterpene. The biosynthesis of terpenes was discussed in a "Real Life" page on isoprene last semester.
Giving the aldehyde shown above the name vitamin A is somewhat deceiving since this term actually refers to an entire family of similar compounds called retinoids, genric structure shown below. Here, R is a carbon that carries an oxygen-containing functionality. The retinoid familty includes retinol, retinal etc. Retinol is often called the pre-formed vitamin a, since it can readily be converted into retinal, retanoic acid etc.
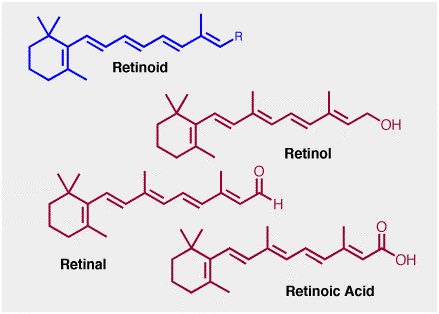
Strictly speaking, the retinal shown is properly described as "all-trans" retinal, since isomerization around one of the central double bonds is a critical step in the chemistry of vision.