
Fatty Acids as an Energy Source
Fats store energy. They store more energy than carbohydrates or proteins, which is why your body keeps them in reserve. Fats are stored as triesters, which when hydrolyzed form the tri-alcohol glycerol and three "free" fatty acids. The acids that are liberated usually have long carbon chains that contain anywhere from 4 to 18 carbons. There are a very large number of fatty acids, the example below shows a fat molecule that consists of three molecules of the fatty acid Palmitic acid (which has a 16 total carbons and is a constituent of palm oil) combined with glycerol. Molecules of this kind are called triglycerides.

Hydrolysis of a triglyceride generates the free fatty acid (3 of them, actually), and it is the further reactions of these free fatty acids that are used by the body to liberate useful energy.
The free fatty acid is first attached to a coenzyme A structure, as shown below (AoCS-CO-R). Note that we are now in the realm of biochemistry, and biochemical mechanisms are not like those in organic chemistry, we can't go into details here. Note also that biomolecules tend to be kind of large and at first site rather complex looking. This is one of the costs of doing chemistry without using the highly reactive reagents that are used in conventional organic chemistry and without having to reflux everything!

The Fatty acid-coenzyme A then undergoes a series of reactions that we can certainly understand, but that use biochemcial mechanisms and "reagent equivalents", and so we won't discuss the details, called beta-oxidation, since oxidation happens at the carbon that is beta to the one that carries the carboxylic acid. The final result is carbon-carbon bond cleavage to generate at acetyl-coenzyme A, AoCS-CO-CH3, that has the coenzyme A plus 2 carbon atoms in the form of an acetyl group (-CO-CH3). This structure then goes off to join the citric acid cycle that generates energy that organisms can use in the form of ATP.
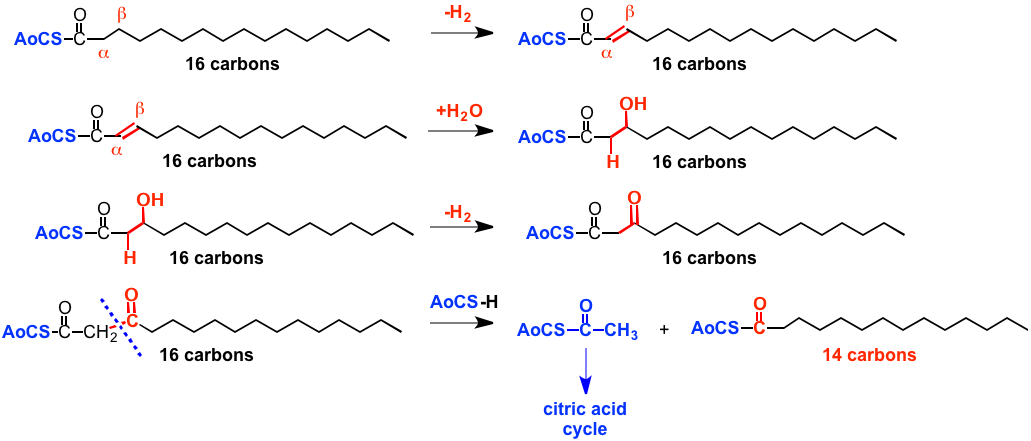
ALSO a product of this series of reactions is ANOTHER AoCS-CO-R that is just 2 carbons shorter than the original free fatty acid, that can then undergo this series of reactions again to generate another AoCS-CO-CH3 and another acid that is shorter by another 2 carbons, which reacts again etc. In this way the entire alkyl chain of the acid is chopped down 2 carbon atoms at a time to generate smaller structures that are used to make energy.
That was a lot of biochemistry which is hard for us to understand at this time, but there is also another point, because when writing this it occurred to me that that your body kind of does exactly the same thing that car does, sort of! Your body likes to get energy from fats rather than, for example carbohydrates, because the energy content of fats is high, because fats are mostly hydrocarbons, just C-C and C-H bonds. Carbohydrates, for example, contain stronger C-O and O-H bonds, and stronger bonds have lower energy electrons, which is one reason why carbohydrates have less energy content than hydrocarbons. And so your car prefers hydrocarbons such as gasoline rather that carbohydrates that plants grow, which is one problem with biofuels, they have too many oxygen atoms in them to be ideal carbon neutral fuels.
And so to make good fuels from biomass, chemical methods of stripping out the oxygen atoms are needed, which is one of the things we are working on my my lab!